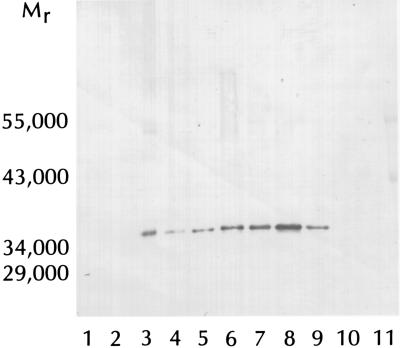
Figure 2.
Western blot analysis of GAPDH incubated with tTGase and casein or CGG. The reaction mixture (25 μl) contained 10 mM Tris⋅HCl buffer (pH 7.2), 10 mM DTT, 10 mM CaCl2, and CGG or protein additions as indicated. After incubation for 0 or 90 min at 20°C, aliquots were withdrawn for determination of GAPDH activity and for SDS/PAGE immunoblotting analysis of GAPDH monomer. GAPDH activity was determined in duplicate 2-μl aliquots. To the remainder (21 μl) was added 5 μl of stop solution containing 60 mM Tris⋅HCl buffer (pH 6.8), 25% (vol/vol) glycerol, 14.4 mM 2-mercaptoethanol, and 0.14% (wt/vol) bromothymol blue. After boiling for 10 min, 20 μl was subjected to SDS/PAGE followed by Coomassie blue staining (not shown), and 5 μl was subjected to SDS/PAGE in a parallel gel followed by Western blotting. Lane 1, 6 milliunits guinea pig liver tTGase (1.6 units/mg), zero time; lane 2, 6 milliunits tTGase, 90 min; lane 3, GAPDH standard (0.6 μg), 90 min; lane 4, GAPDH standard (0.3 μg), 90 min; lane 5, GAPDH + 6 milliunits tTGase, 90 min; lane 6, GAPDH + 50 mM CGG, 90 min; lane 7, GAPDH + CGG + 6 milliunits tTGase, 90 min; lane 8, GAPDH + casein (100 μg), 90 min; lane 9, GAPDH + casein + 6 milliunits tTGase, 90 min; lane 10, casein, zero time; lane 11, casein, 90 min. Mr markers in lane 12 (not shown) were carbonic anhydrase (29,000), lactate dehydrogenase (34,000), ovalbumin (43,000), and glutamate dehydrogenase (55,000) (Diversified Biotech). Lanes 5–9 contained 0.6 μg of GAPDH. Relative activities (%) of GAPDH applied to lanes 3, 5–9 were [100 ± 0], 75 ± 1, 83 ± 3, 10 ± 2, 106 ± 4, and 8 ± 2, respectively (n = three separate experiments carried out in duplicate).