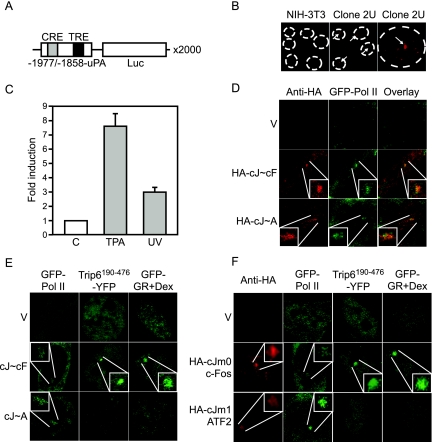
Figure 3.
nTrip6 and GR Are Not Tethered to c-Jun:ATF2-Occupied Promoter
A, Schematic representation of the c-Jun:c-Fos- and c-Jun:ATF2-regulated gene unit amplified in the clone 2U. B, Clone 2U and parental NIH-3T3 fibroblasts were subjected to DNA in situ hybridization using a fluorescently labeled cDNA probe complementary to the luciferase coding sequence. A single gene array is visible in 100% of the 2U cells. C, The uPA gene array is functional. 2U cells were treated with either solvent alone (C) or TPA or UV irradiated. Luciferase activities were determined 6 h later and are plotted as fold induction (mean ± sd of one representative experiment performed in triplicate). D, AP-1 is functionally recruited to the uPA gene array. 2U cells were transfected with GFP-Pol II, together with the empty vector (V), an expression vector for an HA-tagged c-Jun∼c-Fos single chain (HA-cJ∼cF), or an expression vector for an HA-tagged c-Jun∼ATF2 single chain (HA-cJ∼A). The localization of the AP-1 single-chain proteins was determined by immunofluorescence using an anti-HA antibody. The enrichment of the AP-1 proteins and of GFP-Pol II was observed in 70–80% of the cotransfected cells. E and F, nTrip6 and GR are not tethered to c-Jun:ATF2 occupied promoter. E, 2U cells were transfected with empty vector, an expression vector for c-Jun∼c-Fos single chain (cJ∼cF), or an expression vector for c-Jun∼ATF2 single chain (cJ∼A), together with an expression vector for GFP-Pol II, an expression vector for Trip6190–476 fused to YFP (YFP-Trip6190–476), or an expression vector for GR fused to GFP. Cells were treated with dexamethasone for 30 min and imaged by confocal microscopy. Nuclei of representative cells are shown. F, Clone 2U uPA gene array cells were cotransfected with either GFP-Pol II, Trip6190–476 fused to YFP (YFP-Trip6190–476), or GR fused to GFP, together with empty vector (V), HA-tagged c-Jun-m0 mutant (HA-cJm0, c-Fos seeking) and c-Fos, or HA-tagged c-Jun-m1 mutant (HA-cJm1, ATF2 seeking) and ATF2. Cells were treated with dexamethasone for 30 min (Dex). The localization of the c-Jun mutants was determined by immunofluorescence using an anti-HA antibody. Cells were imaged by confocal microscopy. Nuclei of representative cells are shown.