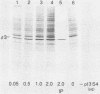
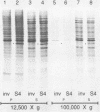
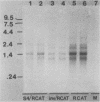
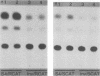
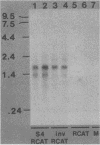
Abstract
The mammalian reovirus S4 gene has been implicated in the serotype-dependent inhibition of host cell protein synthesis during viral replication in mouse L cells. To examine the effect(s) of this gene on transcription or translation or both, a DNA copy of the serotype 3 S4 gene was inserted into a eucaryotic expression vector. Cotransfection of COS cells with plasmids containing S4 and the reporter gene, chloramphenicol acetyltransferase (CAT), resulted in a marked stimulation of CAT expression, predominantly at the level of translation. The significance of these findings is discussed in relation to the double-stranded-RNA-binding activity of the S4 gene product, polypeptide sigma 3.
Full text
PDF
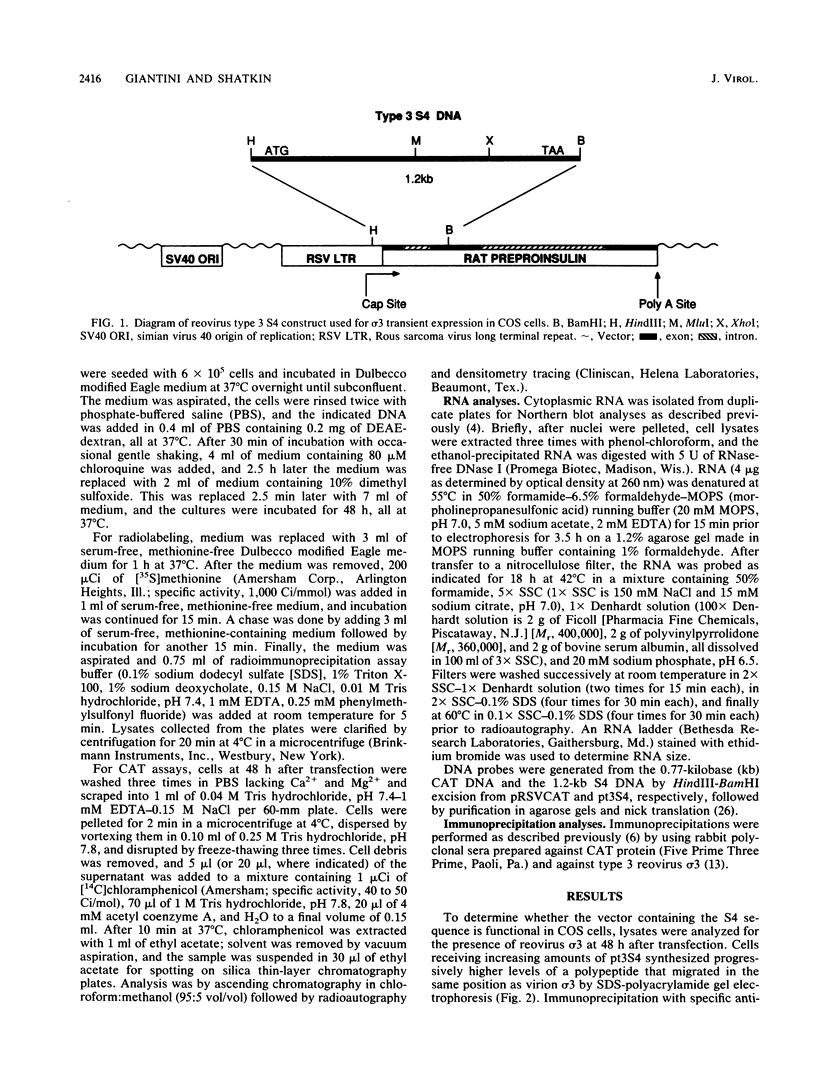
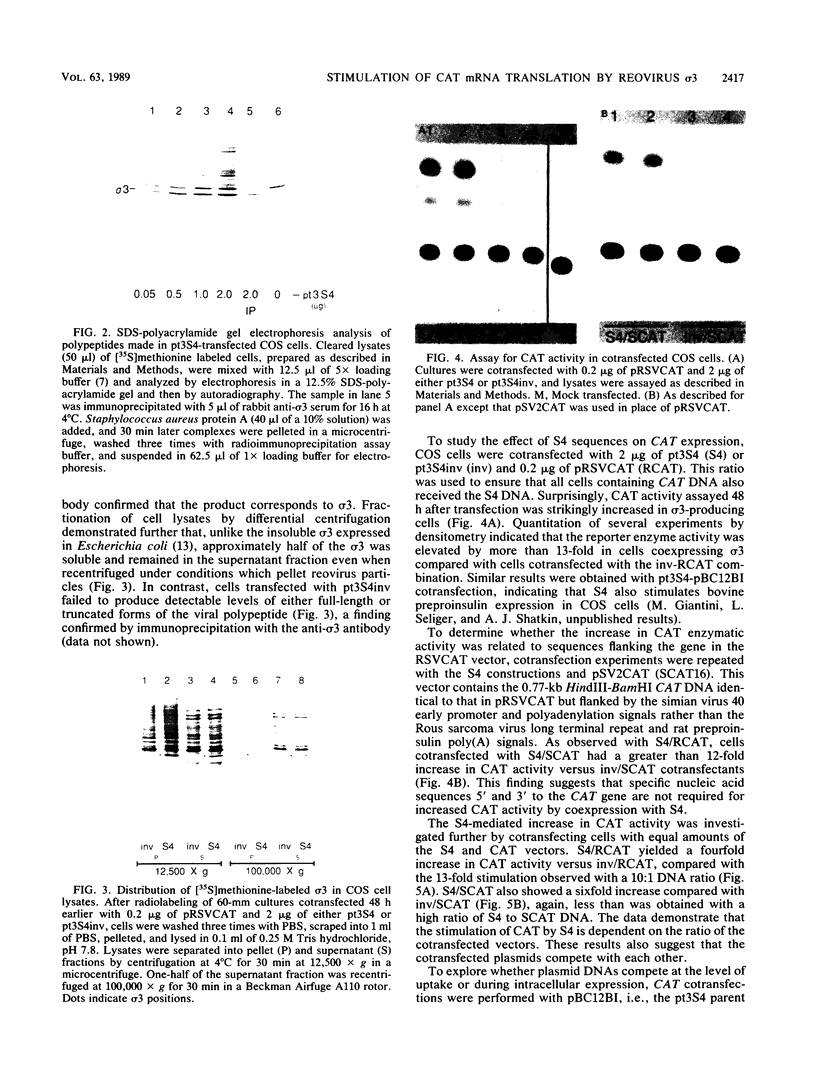
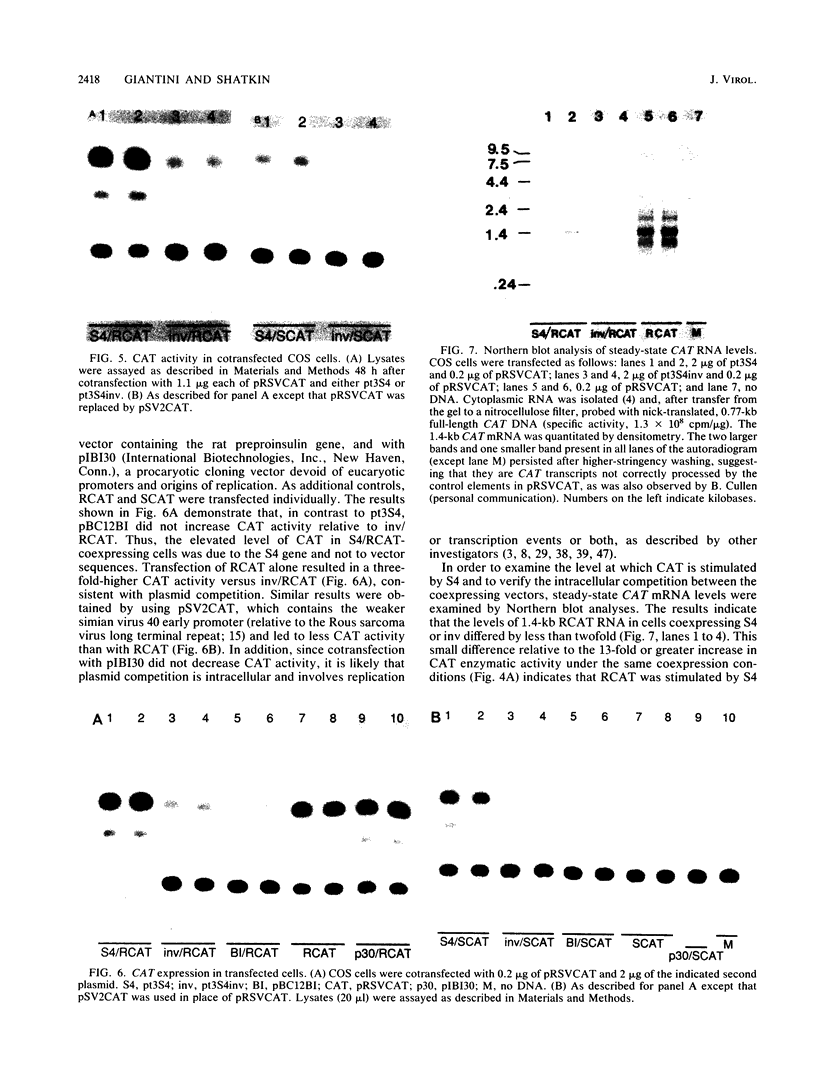


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Mathews M. B., Andersson P., Vennström B., Pettersson U. Structure of genes for virus-associated RNAI and RNAII of adenovirus type 2. Proc Natl Acad Sci U S A. 1980 May;77(5):2424–2428. doi: 10.1073/pnas.77.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akusjärvi G., Svensson C., Nygård O. A mechanism by which adenovirus virus-associated RNAI controls translation in a transient expression assay. Mol Cell Biol. 1987 Jan;7(1):549–551. doi: 10.1128/mcb.7.1.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo S., Yun M., Beemon K. cis-acting regulatory elements within gag genes of avian retroviruses. Mol Cell Biol. 1987 Jan;7(1):388–397. doi: 10.1128/mcb.7.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R., Maguire J. E., Singer D. S. Identification of negative and positive regulatory elements associated with a class I major histocompatibility complex gene. Mol Cell Biol. 1988 Feb;8(2):695–703. doi: 10.1128/mcb.8.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduchi E., Esteban M., Carrasco L. Reovirus type 3 synthesizes proteins in interferon-treated HeLa cells without reversing the antiviral state. Virology. 1988 Jun;164(2):420–426. doi: 10.1016/0042-6822(88)90555-7. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., McMenamin M. M. Early virion-associated suppression of cellular protein synthesis by herpes simplex virus is accompanied by inactivation of mRNA. J Gen Virol. 1984 Jul;65(Pt 7):1225–1228. doi: 10.1099/0022-1317-65-7-1225. [DOI] [PubMed] [Google Scholar]
- George C. X., Crowe A., Munemitsu S. M., Atwater J. A., Samuel C. E. Biosynthesis of reovirus-specified polypeptides. Molecular cDNA cloning and nucleotide sequence of the reovirus serotype 1 Lang strain s2 mRNA which encodes the virion core polypeptide sigma 2. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1153–1161. doi: 10.1016/s0006-291x(87)80190-0. [DOI] [PubMed] [Google Scholar]
- Giantini M., Seliger L. S., Furuichi Y., Shatkin A. J. Reovirus type 3 genome segment S4: nucleotide sequence of the gene encoding a major virion surface protein. J Virol. 1984 Dec;52(3):984–987. doi: 10.1128/jvi.52.3.984-987.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giantini M., Shatkin A. J. Reovirus major capsid protein expressed in Escherichia coli. Gene. 1987;56(1):153–160. doi: 10.1016/0378-1119(87)90168-5. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R., Tamm I. Initiation of DNA replication in mammalian cells and its inhibition by reovirus infection. J Mol Biol. 1974 Jan 15;82(2):175–183. doi: 10.1016/0022-2836(74)90339-8. [DOI] [PubMed] [Google Scholar]
- Huismans H., Joklik W. K. Reovirus-coded polypeptides in infected cells: isolation of two native monomeric polypeptides with affinity for single-stranded and double-stranded RNA, respectively. Virology. 1976 Apr;70(2):411–424. doi: 10.1016/0042-6822(76)90282-8. [DOI] [PubMed] [Google Scholar]
- Imani F., Jacobs B. L. Inhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 sigma 3 protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7887–7891. doi: 10.1073/pnas.85.21.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P. Translational control mediated by eucaryotic initiation factor-2 is restricted to specific mRNAs in transfected cells. Mol Cell Biol. 1987 Apr;7(4):1568–1571. doi: 10.1128/mcb.7.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S., Simizu B., Ishida I., Oya A., Yamada M. Inhibition of DNA synthesis in BHK cells infected with western equine encephalitis virus. 2. Properties of the inhibitory factor of DNA polymerase induced in infected cells. Virology. 1979 Oct 30;98(2):439–447. doi: 10.1016/0042-6822(79)90566-x. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology. 1981 Jan 15;108(1):134–146. doi: 10.1016/0042-6822(81)90533-x. [DOI] [PubMed] [Google Scholar]
- Lemieux R., Lemay G., Millward S. The viral protein sigma 3 participates in translation of late viral mRNA in reovirus-infected L cells. J Virol. 1987 Aug;61(8):2472–2479. doi: 10.1128/jvi.61.8.2472-2479.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran A., Mathews M. B. Characterization of the double-stranded RNA implicated in the inhibition of protein synthesis in cells infected with a mutant adenovirus defective for VA RNA. Virology. 1988 May;164(1):106–113. doi: 10.1016/0042-6822(88)90625-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mercola M., Goverman J., Mirell C., Calame K. Immunoglobulin heavy-chain enhancer requires one or more tissue-specific factors. Science. 1985 Jan 18;227(4684):266–270. doi: 10.1126/science.3917575. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Degradation of cellular mRNA during infection by herpes simplex virus. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2370–2374. doi: 10.1073/pnas.74.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacha R. F., Condit R. C. Characterization of a temperature-sensitive mutant of vaccinia virus reveals a novel function that prevents virus-induced breakdown of RNA. J Virol. 1985 Nov;56(2):395–403. doi: 10.1128/jvi.56.2.395-403.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensiero M. N., Lucas-Lenard J. M. Evidence for the presence of an inhibitor on ribosomes in mouse L cells infected with mengovirus. J Virol. 1985 Oct;56(1):161–171. doi: 10.1128/jvi.56.1.161-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Granoff A. Cell-free translation of frog virus 3 messenger RNAs. Initiation factors from infected cells discriminate between early and late viral mRNAs. J Biol Chem. 1983 Jan 10;258(1):571–578. [PubMed] [Google Scholar]
- Rice A. P., Roberts B. E. Vaccinia virus induces cellular mRNA degradation. J Virol. 1983 Sep;47(3):529–539. doi: 10.1128/jvi.47.3.529-539.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Goh W. C., Dayton A. I., Lippke J., Haseltine W. A. Post-transcriptional regulation accounts for the trans-activation of the human T-lymphotropic virus type III. Nature. 1986 Feb 13;319(6054):555–559. doi: 10.1038/319555a0. [DOI] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Co M. S., Brown E. G., Fields B. N. Distinct binding sites for zinc and double-stranded RNA in the reovirus outer capsid protein sigma 3. Mol Cell Biol. 1988 Jan;8(1):273–283. doi: 10.1128/mcb.8.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Shenk T. Impact of virus infection on host cell protein synthesis. Annu Rev Biochem. 1987;56:317–332. doi: 10.1146/annurev.bi.56.070187.001533. [DOI] [PubMed] [Google Scholar]
- Scholer H., Haslinger A., Heguy A., Holtgreve H., Karin M. In vivo competition between a metallothionein regulatory element and the SV40 enhancer. Science. 1986 Apr 4;232(4746):76–80. doi: 10.1126/science.3006253. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Reovirus inhibition of cellular RNA and protein synthesis: role of the S4 gene. Virology. 1982 Oct 30;122(2):381–391. doi: 10.1016/0042-6822(82)90237-9. [DOI] [PubMed] [Google Scholar]
- Skup D., Millward S. Reovirus-induced modification of cap-dependent translation in infected L cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):152–156. doi: 10.1073/pnas.77.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N. Regulation of translation by poliovirus. Adv Virus Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Adenovirus VA RNAI mediates a translational stimulation which is not restricted to the viral mRNAs. EMBO J. 1985 Apr;4(4):957–964. doi: 10.1002/j.1460-2075.1985.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. E., Godefroy-Colburn T., Thach R. E. The role of mRNA competition in regulating translation. I. Demonstration of competition in vivo. J Biol Chem. 1981 Nov 25;256(22):11739–11746. [PubMed] [Google Scholar]
- Weinmann R., Raskas H. J., Roeder R. G. Role of DNA-dependent RNA polymerases II and III in transcription of the adenovirus genome late in productive infection. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3426–3439. doi: 10.1073/pnas.71.9.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Cheng P. F., Conrad K. Expression of transfected DNA depends on DNA topology. Cell. 1986 Jul 4;46(1):115–122. doi: 10.1016/0092-8674(86)90865-2. [DOI] [PubMed] [Google Scholar]
- Willems M., Penman S. The mechanism of host cell protein synthesis inhibition by poliovirus. Virology. 1966 Nov;30(3):355–367. doi: 10.1016/0042-6822(66)90114-0. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]