Abstract
Stimulus recognition in monkeys is severely impaired by destruction or dysfunction of the perirhinal cortex and also by systemic administration of the cholinergic-muscarinic receptor blocker, scopolamine. These two effects are shown here to be linked: Stimulus recognition was found to be significantly impaired after bilateral microinjection of scopolamine directly into the perirhinal cortex, but not after equivalent injections into the laterally adjacent visual area TE or into the dentate gyrus of the overlying hippocampal formation. The results suggest that the formation of stimulus memories depends critically on cholinergic-muscarinic activation of the perirhinal area, providing a new clue to how stimulus representations are stored.
Keywords: memory, recognition, rhinal cortex, hippocampus, cholinergic-muscarinic transmission
Stimulus recognition is a basic form of memory that depends on interaction between the limbic system and the cortical sensory streams that process stimulus quality (1–4). The relevant stream for vision is the multisynaptic occipitotemporal pathway, and a particularly important sector for recognition is its final station, inferior temporal cortical visual area (TE) (5), which is also the pathway’s major source of projections to the limbic system (6). The major limbic recipient of these projections, as well as those from stimulus processing streams in other modalities, is the perirhinal cortex (PR, ref. 7), an area now known to be more important than the hippocampus for recognition memory (8–14). How TE-PR interaction results in visual recognition is unknown, but because the muscarinic antagonist, scopolamine, impairs visual recognition, and the cholinesterase inhibitor, physostigmine, improves it (15–17), as they do many other forms of memory (for review, see ref. 17), a critical factor could be cholinergic modulation of neuronal activity in the ventral temporal region. Other neuromodulators of activity in this region are far less likely to be important for visual recognition, inasmuch as systemic injections of noradrenergic, dopaminergic, and serotonergic antagonists have been found to leave such memory unaffected (T.G.A., unpublished observations). To determine whether local cerebral cholinergic modulation is indeed critical, we examined the effects of direct ventral temporal injections of scopolamine in monkeys performing a visual recognition task.
MATERIALS AND METHODS
Animals.
Three male rhesus monkeys (Macaca mulatta, each weighing about 6.5 kg at the beginning of the study) were housed in individual cages in rooms with automatically regulated lighting (light/dark, 12:12 h) and maintained on a diet of monkey chow supplemented with fruit. Water was always available in the home cage. During behavioral training, food rations were regulated to ensure maximum feeding consistent with prompt responding in the testing apparatus. This study was conducted under a protocol approved by the National Institute of Mental Health Animal Care and Use Committee and in accord with The Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
Apparatus and Behavioral Procedure.
The monkeys were tested in a darkened, sound-attenuated cubicle while seated in a primate chair facing a color monitor with a touch-sensitive screen. They were pretrained on a computerized version of delayed nonmatching-to-sample (DNMS) with trial-unique stimuli and gradually shaped until they could perform this recognition task with list lengths of 20 sample stimuli (18). In the sample phase of the task, 20 stimuli drawn randomly from a supply of 1,200 colored graphic symbols were presented in succession at 30-sec intervals in the center of the monitor, and the monkey obtained a banana pellet upon touching each stimulus in turn. In the choice phase, the 20 sample stimuli were presented again in the same order and at the same rate (i.e., 30-sec intervals) as before, but now pseudorandomly on the left or right of the monitor, each paired with a new stimulus appearing in the other lateral position; in this choice phase, the animal was rewarded with a banana pellet for touching the new stimulus in each pair. The test thus took 20 min to complete and measured the animal’s ability to recognize each sample stimulus 10 min after its initial presentation. The monkeys were assessed on four such lists each day, during an 80-min daily session, 5 days per week.
Surgery.
After DNMS training and before testing, the three monkeys received surgically implanted plastic cylinders (18) for microinjection access. Stereotaxic coordinates for the implants were derived from preoperative MRI scans (19). For implantation surgery, the animal was intubated and deeply anesthetized with isoflurane, after which, under aseptic conditions, the skin, connective tissue, and muscle were incised and retracted, and a trephine opening was made on each side of the skull. The openings were 18 mm in diameter and were centered approximately 13 mm anterior to the interaural plane and 14 mm lateral to the midline. The plastic cylinders, 21 mm OD and 18 mm ID, were then affixed to the openings with resin cement, and the soft tissues were sutured around the cylinder, which was then closed with a removable, tight-fitting, plastic cap. After a one-week recovery period, the monkeys resumed daily testing as before.
Intracerebral Injection.
Once each week, immediately before that day’s test session, the monkeys received simultaneous microinjections into symmetrical areas of the two hemispheres. Stereotaxic coordinates for the targeted cerebral areas were calculated on the basis of postoperative MRI scans (19). The injections were directed into either PR, TE (near the border with PR), or the dentate gyrus (DG) of the hippocampus (Fig. 1 A and B); these three areas were targeted in rotation on successive weeks. The injectant consisted of 3 μl of 0 (saline control), 1, 5, or 10 mM of scopolamine solution, administered in that order (i.e., first the saline control solution was infused bilaterally into each of the three areas on successive weeks, then the 1 mM solution was infused into each, etc.). Scopolamine hydrobromide (Sigma) was freshly dissolved in sterile saline to concentrations of 1, 5, or 10 mM, and filtered through 0.22-μm sterile filters (Millipore) immediately before the injections. With the monkey seated in the testing cubicle, the cylinders and surrounding tissue were first thoroughly cleaned with 3% hydrogen peroxide. A specially designed three-dimensional micromanipulator was then attached to each cylinder, and the injection needle (0.3 mm OD and 0.15 mm ID), which had been disinfected by overnight storage in 70% ethyl alcohol, was inserted into the targeted cerebral area by the micromanipulator. Delivery of the injectant on each side was controlled by a precision microinjection pump (CMA/100, CMA/Microdialysis) and took 20 min to complete (3 μl at the rate of 0.15 μl/min). DNMS testing was begun immediately thereafter.
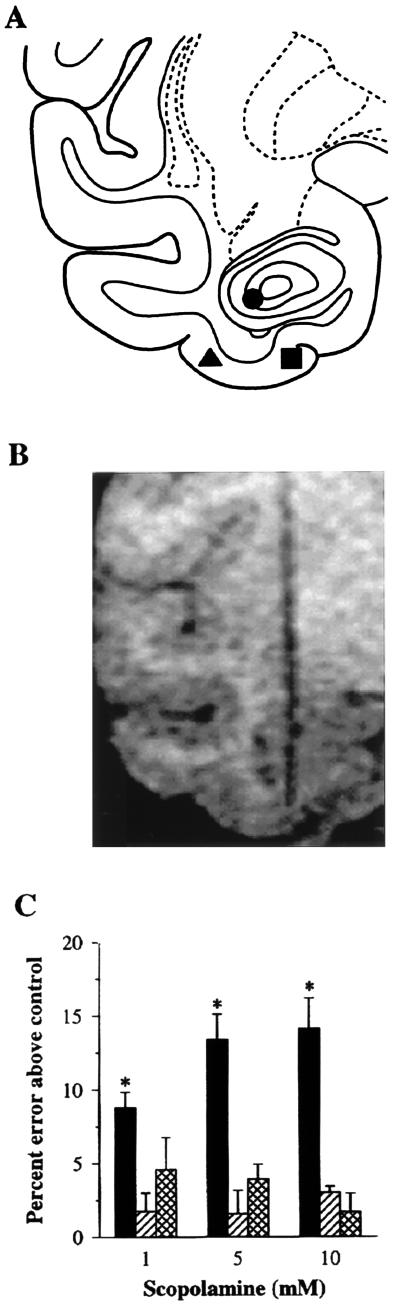
Figure 1.
Loci of injections and effects on DNMS performance. (A) Injection loci in PR (▪), cortical visual area TE (▴), and the DG of the hippocampus (•). Stereotaxic coordinates for these targeted cerebral areas were calculated on the basis of postoperative MRI scans. Such MRI-guided stereotaxic placement of probes was shown (19) to be highly precise, histologically verified endpoints (n = 42) deviating from the intended targets a maximum of 0.5 mm in both the parasagittal and horizontal planes and 0.7 mm in the coronal plane, for a maximal error of 1.0 mm considering all three planes simultaneously. (B) MRI scan illustrating an MRI-guided stereotaxic probe lowered into PR; the probe consists of fused silica tubing containing gadolinium solution in place of the injection needle. (C) DNMS performance after bilateral scopolamine injections into the three areas (PR, black bars; TE, striped bars; and DG, crosshatched bars). Baseline error rates after the saline control injections (indicated as 0 on the ordinate) were: PR, 22.9%; TE, 22.0%; and DG, 22.9%. Values shown are mean percent errors (±SE) above these baseline values produced by each of the three doses of scopolamine (1, 5, and 10 mM solutions). ∗, P < 0.05 vs. the saline control, based on the Bonferroni method following a two-way repeated measures ANOVA (F6,12 = 12.0, P < 0.001); the effects of the three different doses in PR did not differ significantly from each other. Statistical results were the same when the average score for three areas (22.6%) for the saline-control injections was used as the baseline error rate.
Statistical Analyses.
The results were subjected to one-way (area or dose) and two-way (area by dose) repeated measures ANOVA, followed by interdose and interarea comparisons by the Bonferroni method, using sigmastat 1.0 (Jandel).
RESULTS AND DISCUSSION
On noninjection days, the monkeys scored an average of 78.2% correct responses. This level of performance, which is about the same as that found (15, 16, 20) in other monkeys tested on DNMS with a list length of 20 sample stimuli, was not altered significantly by bilateral infusion of saline alone into any of the three cerebral areas (PR, 77.1%; TE, 78.0%; DG, 77.1%). Performance levels fell, however, when the different doses of scopolamine were infused; the reductions from the saline control scores, averaged across the three doses in each area, were: PR, 12.1%; TE, 2.1%; and DG, 3.4%. The average decrease in performance level after the perirhinal injections was significant (one-way repeated measures ANOVA, F3,6 = 26.6, P < 0.001), as were the decreases after each of the three doses (1, 5, and 10 mM) considered separately (Fig. 1C). Furthermore, the scopolamine injections into PR lowered scores significantly compared with the scores after the scopolamine injections into TE and DG (two-way repeated measures ANOVA, F2,4 = 55.9, P < 0.001; PR vs. TE and PR vs. DG, P < 0.05 for each). By contrast, the effects of the TE and DG injections (which were placed about 5 mm lateral and dorsolateral, respectively, to those in PR) were not significant (Fig. 1C). These differential findings set an upper limit for diffusion of the drug between the effective and ineffective sites. All of the above comparisons yielded the same statistical results when the average of the saline control scores for the three areas (77.4%) was substituted for the saline control scores calculated for each area separately.
Although the lack of significant impairment following the injections into TE and DG does not rule out a contribution to memory from cholinergic-muscarinic activation of these areas, the differential results are directly in line with those from lesion studies, which have shown that visual recognition is impaired far more by perirhinal ablations than by equally extensive lesions elsewhere in the temporal lobes, including TE (21) and DG (8–14).
The impairment in recognition accuracy after the bilateral perirhinal injections did not vary significantly as a function of list position (lists 1–4) within the daily sessions, and it was not accompanied by significant alterations in either response latency (saline, 3.6 ± 1.0 sec; scopolamine, 3.5 ± 0.9 sec) or position bias, measured as the difference between left correct and right correct divided by their sum (saline, 0.08 ± 0.01; scopolamine, 0.10 ± 0.03). Also, unlike the recognition deficits produced by systemic administration of scopolamine (15, 16), those produced by the perirhinal injections did not vary significantly as a function of dose, although there was a trend in this direction. A reliable dose-dependent deficit might have required testing with a wider range of drug concentrations. Alternatively, intracerebral scopolamine in a given area may be a function not of drug concentration but of the proportion of the area infused by an effective concentration, in which case greater deficits than those seen here would be expected to follow only more extensive perirhinal infusions.
A neuronal correlate of stimulus repetition has been identified in monkey PR. When a stimulus is presented as the match in a serial, delayed matching-to-sample task (22–27), many cells in PR and surrounding cortex discharge significantly less to the repetition of that stimulus than to its original presentation as the sample. Such “repetition suppression” is stimulus specific, is unaffected by other intervening stimuli, and occurs to the same degree whether the stimulus is highly familiar or initially novel (26), qualifying it as a candidate mechanism for mediating stimulus recognition. However, monkeys tested on a serial delayed matching-to-sample task while under the effects of systemically administered scopolamine showed the expected decrement in performance accuracy even though the repetition suppression of perirhinal neuronal activity remained entirely normal (28). The drug-induced dissociation between memory scores and neuronal activity led to the conclusion that scopolamine either acts entirely outside PR or disrupts some other, critical, mechanism within PR (28). By ruling out the first alternative, the present results provide strong support for the second.
The identity of the critical neuronal mechanism is unknown, but the present and a previous scopolamine study provide two important clues. First, the earlier study (29) showed that recognition is impaired when scopolamine is administered before stimulus samples are presented for familiarization, though not when the same doses of the drug are given after stimulus familiarization but before the choice tests, suggesting that scopolamine interferes with the storage of stimulus representations but not with their later retrieval. Second, as shown here, the putative storage process is at least partly dependent on cholinergic activation of muscarinic receptors located on perirhinal neurons, specifically. Because the storage of stimulus representations is basic not only to stimulus recognition, but also to the many stimulus memory processes that build on recognition, such as recency memory, working memory, serial-order memory, and associative memory, including associative recognition and recall, elucidation of the storage process would have far-reaching consequences. A valuable step in that direction would be identification of the muscarinic-dependent, perirhinal neuronal correlate of stimulus familiarization.
Acknowledgments
We thank Robert Desimone for valuable comments on the manuscript.
ABBREVIATIONS
- DG
dentate gyrus
- DNMS
delayed nonmatching-to-sample
- PR
perirhinal cortex
- TE
inferior temporal cortical visual area
References
- 1.Murray E A, Mishkin M. J Neurosci. 1984;4:2565–2580. doi: 10.1523/JNEUROSCI.04-10-02565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishkin M, Phillips R R. In: Brain Circuits and Functions of the Mind: Essays in Honor of Roger W. Sperry. Trevarthen C, editor. London: Cambridge Univ. Press; 1990. pp. 196–210. [Google Scholar]
- 3.Otto T, Eichenbaum H. Behav Neurosci. 1992;106:762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki W A, Zola-Morgan S, Squire L R, Amaral D G. J Neurosci. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishkin M. Philos Trans R Soc London B. 1982;298:85–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki W A, Amaral D G. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki W A, Amaral D G. J Neurosci. 1994;14:1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horel J A, Voytko M L, Salsbury K G. Behav Neurosci. 1984;98:310–324. doi: 10.1037//0735-7044.98.2.310. [DOI] [PubMed] [Google Scholar]
- 9.Horel J A, Pytko-Joiner D E, Voytko M L, Salsbury K. Behav Brain Res. 1987;23:29–42. doi: 10.1016/0166-4328(87)90240-3. [DOI] [PubMed] [Google Scholar]
- 10.Zola-Morgan S, Squire L R, Amaral D G, Suzuki W. J Neurosci. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meunier M, Bachevalier J, Mishkin M, Murray E A. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishkin M. Nature (London) 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- 13.Clower R P, Alvarez-Royo P, Zola-Morgan S, Squire L R. Soc Neurosci Abstr. 1991;17:338. doi: 10.1016/0165-0270(91)90172-v. [DOI] [PubMed] [Google Scholar]
- 14.Murray E A, Mishkin M. Soc Neurosci Abstr. 1996;22:281. [Google Scholar]
- 15.Aigner T G, Mishkin M. Behav Neural Biol. 1986;45:81–87. doi: 10.1016/s0163-1047(86)80008-5. [DOI] [PubMed] [Google Scholar]
- 16.Ogura H, Aigner T G. J Pharmacol Exp Ther. 1993;266:60–64. [PubMed] [Google Scholar]
- 17.Hasselmo M E. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Aigner T G. NeuroReport. 1996;7:2231–2235. doi: 10.1097/00001756-199609020-00034. [DOI] [PubMed] [Google Scholar]
- 19.Saunders R C, Aigner T G, Frank J A. Exp Brain Res. 1990;81:443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka N, Aigner T G. NeuroReport. 1996;7:565–568. doi: 10.1097/00001756-199601310-00045. [DOI] [PubMed] [Google Scholar]
- 21.Buckley M J, Gaffan D, Murray E A. J Neurophysiol. 1997;77:587–598. doi: 10.1152/jn.1997.77.2.587. [DOI] [PubMed] [Google Scholar]
- 22.Riches I P, Wilson F A W, Brown M W. J Neurosci. 1991;11:1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller E K, Li L, Desimone R. Science. 1991;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- 24.Miller E K, Li L, Desimone R. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahy F L, Riches I P, Brown M W. Exp Brain Res. 1993;96:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Miller E K, Desimone R. J Neurophysiol. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- 27.Lueschow A, Miller E K, Desimone R. Cereb Cortex. 1994;4:523–531. doi: 10.1093/cercor/4.5.523. [DOI] [PubMed] [Google Scholar]
- 28.Miller E K, Desimone R. NeuroReport. 1993;4:81–84. doi: 10.1097/00001756-199301000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Aigner T G, Walker D L, Mishkin M. Behav Neural Biol. 1991;55:61–67. doi: 10.1016/0163-1047(91)80127-z. [DOI] [PubMed] [Google Scholar]