Fig. 1. Metabolism of arylamines includes both activation and inactivation via N-acetylation, O-acetylation, and N,O-acetylation catalyzed by NAT2.
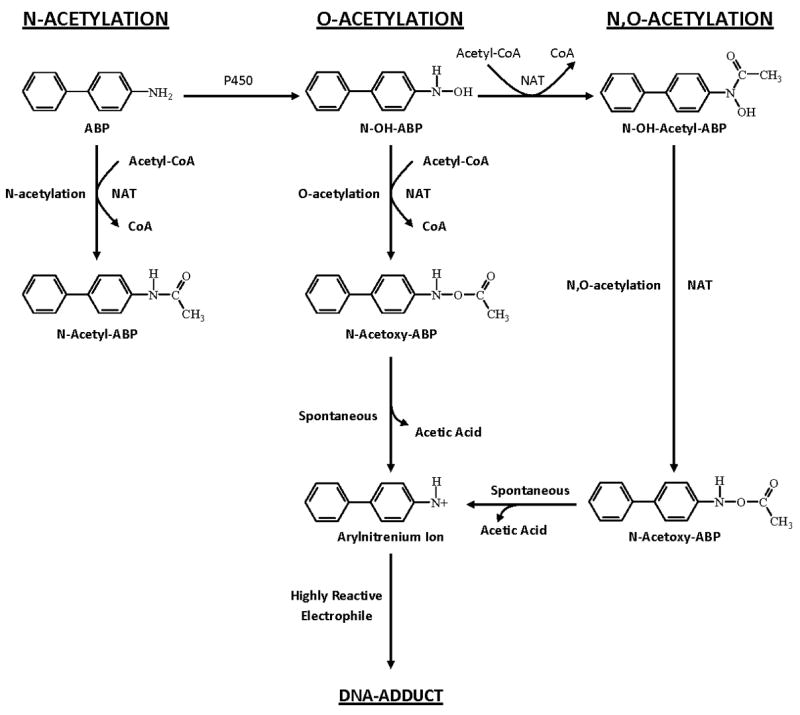
These reactions are depicted for the arylamine carcinogen 4-aminobiphenyl, ultimately leading to the generation of highly reactive electrophiles that bind to DNA potentially leading to mutations and cancer. Adapted from [5].
