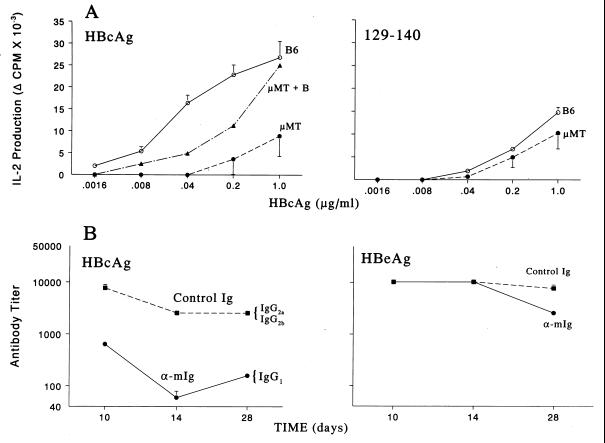
Figure 6.
B cell APC are required for efficient priming of Th cells and antibody production in vivo. (A) Groups of five wild-type B6 or B cell knockout μMT mice were immunized with HBcAg (Left) or peptide 129–140 (Right). Ten days later, draining popliteal lymph node cells were harvested, pooled, and cultured with media or HBcAg (.0016–1.0 μg/ml), and IL-2 levels in 24 h SN were determined by bioassay. In selected experiments unprimed B cells were added to μMT lymph node cells during in vitro culture to provide a source of B cell APC. This experiment was performed on three separate occasions, and the data represent mean values ± SD. [B6 > μMT; P < .05, Mann–Whitney (Left)]. (B) Groups of five (B10 × B10.S)F1 mice were treated with 0.5 mg of goat anti-mIg F(ab′)2 fragments (bivalent) or goat Ig as a control on days 0, 1, and 2, and immunized with HBcAg (.05 μg) in saline (Left) or HBeAg (10 μg) in saline (Right) on day 0. After immunization, sera were collected, pooled, and assayed for anti-HBc or anti-HBe antibodies by solid-phase ELISA (9). The data are expressed as antibody end-point titer representing the highest serum dilution that yielded an OD492 reading three times that of preimmunization sera. This experiment was performed twice, and mean values ± SD are shown.