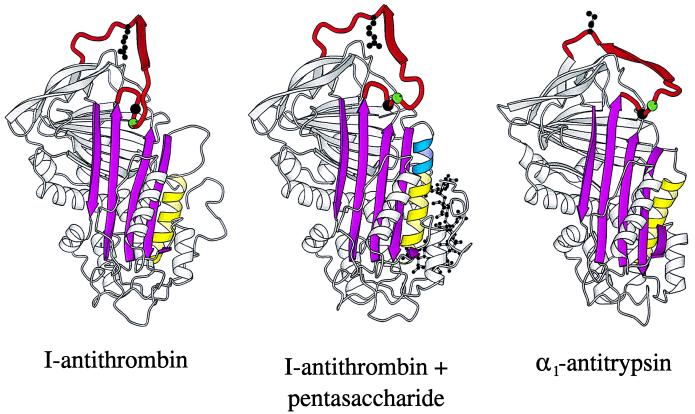
Figure 3.
Ribbon diagrams of (from left) I-antithrombin (15), pentasaccharide-complexed I-antithrombin, and α1-antitrypsin (32). The pentasaccharide activation of I-antithrombin is seen to involve a closing of the A-sheet (magenta), an extension (blue) of helix D (yellow), and an expulsion of residues P14 (green sphere) and P15 (black sphere) of the reactive site loop (red). The reactive loop of both antithrombin molecules is constrained by the dimer contact (see Fig. 2a) of the β-pleated P3–P8 (ribboned arrow). An indication of the likely free conformation, with exposure of the P1 reactive center (shown as a ball–stick model), is provided by the optimal inhibitory conformation of the reactive loop present in α1-antitrypsin (32).