Abstract
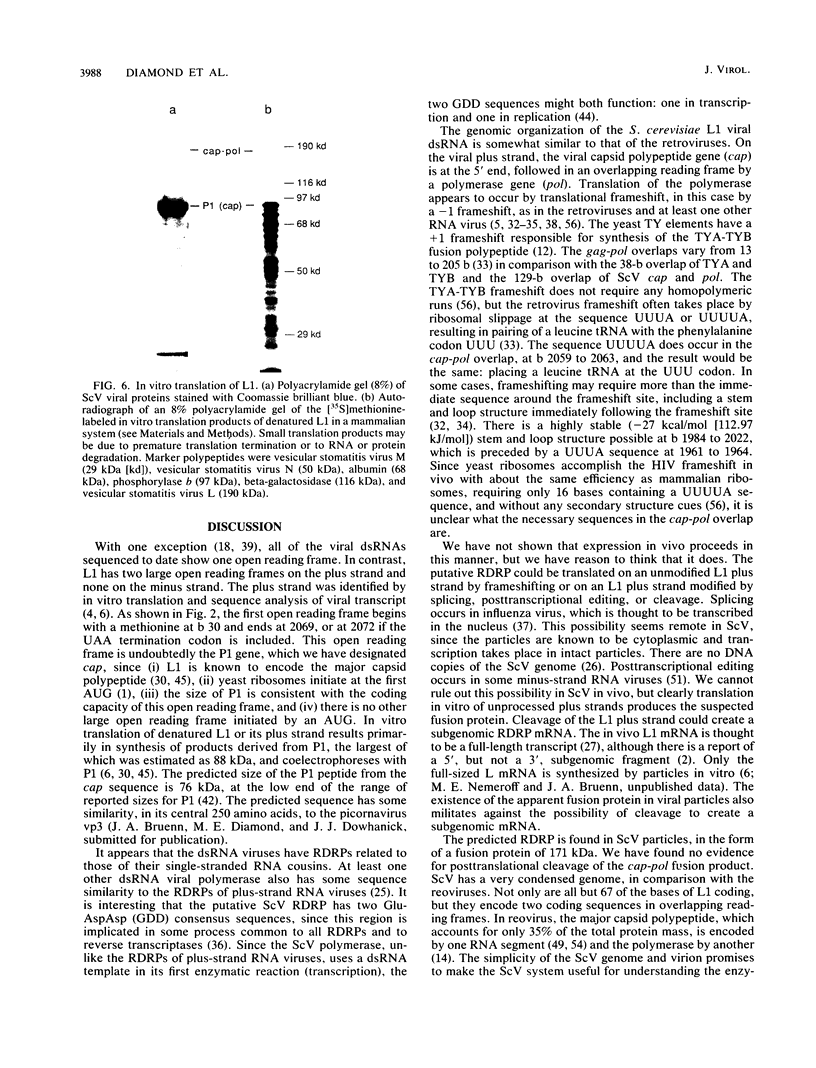
The Saccharomyces cerevisiae viruses have a large viral double-stranded RNA which encodes the major viral capsid polypeptide. We have previously shown that this RNA (L1) also encodes a putative viral RNA-dependent RNA polymerase (D. F. Pietras, M. E. Diamond, and J. A. Bruenn, Nucleic Acids Res., 16:6226, 1988). The organization and expression of the viral genome is similar to that of the gag-pol region of the retroviruses. The complete sequence of L1 demonstrates two large open reading frames on the plus strand which overlap by 129 bases. The first is the gene for the capsid polypeptide, and the second is the gene for the putative RNA polymerase. One of the products of in vitro translation of the denatured viral double-stranded RNA is a polypeptide of the size expected of a capsid-polymerase fusion protein, resulting from a -1 frameshift within the overlapping region. A polypeptide of the size expected for a capsid-polymerase fusion product was found in virions, and it was recognized in Western blots (immunoblots) by antibodies to a synthetic peptide derived from the predicted polymerase sequence.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baim S. B., Sherman F. mRNA structures influencing translation in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1988 Apr;8(4):1591–1601. doi: 10.1128/mcb.8.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Burn V. E., Jayachandran S., Tipper D. J. Yeast killer dsRNA plasmids are transcribed in vivo to produce full and partial-length plus-stranded RNAs. Nucleic Acids Res. 1983 Feb 25;11(4):1077–1097. doi: 10.1093/nar/11.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Brennan V. E., Bobek L. A., Bruenn J. A. Yeast deRNA viral transcriptase pause products: identification of the transcript strand. Nucleic Acids Res. 1981 Oct 10;9(19):5049–5059. doi: 10.1093/nar/9.19.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley I., Boursnell M. E., Binns M. M., Bilimoria B., Blok V. C., Brown T. D., Inglis S. C. An efficient ribosomal frame-shifting signal in the polymerase-encoding region of the coronavirus IBV. EMBO J. 1987 Dec 1;6(12):3779–3785. doi: 10.1002/j.1460-2075.1987.tb02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J. A., Brennan V. E. Yeast viral double-stranded RNAs have heterogeneous 3' termini. Cell. 1980 Apr;19(4):923–933. doi: 10.1016/0092-8674(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A. Virus-like particles of yeast. Annu Rev Microbiol. 1980;34:49–68. doi: 10.1146/annurev.mi.34.100180.000405. [DOI] [PubMed] [Google Scholar]
- Bruenn J., Bobek L., Brennan V., Held W. Yeast viral RNA polymerase is a transcriptase. Nucleic Acids Res. 1980 Jul 11;8(13):2985–2997. doi: 10.1093/nar/8.13.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Kane W. Relatedness of the double-stranded RNAs present in yeast virus-like particles. J Virol. 1978 Jun;26(3):762–772. doi: 10.1128/jvi.26.3.762-772.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Keitz B. The 5' ends of yeast killer factor RNAs are pppGp. Nucleic Acids Res. 1976 Oct;3(10):2427–2436. doi: 10.1093/nar/3.10.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Madura K., Siegel A., Miner Z., Lee M. Long internal inverted repeat in a yeast viral double-stranded RNA. Nucleic Acids Res. 1985 Mar 11;13(5):1575–1591. doi: 10.1093/nar/13.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare J., Farabaugh P. Nucleotide sequence of a yeast Ty element: evidence for an unusual mechanism of gene expression. Proc Natl Acad Sci U S A. 1985 May;82(9):2829–2833. doi: 10.1073/pnas.82.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihanich M., van Tuinen E., Lambris J. D., Marshallsay B. Accumulation of viruslike particles in a yeast mutant lacking a mitochondrial pore protein. Mol Cell Biol. 1989 Mar;9(3):1100–1108. doi: 10.1128/mcb.9.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Activation and characterization of the reovirus transcriptase: genetic analysis. J Virol. 1982 Jan;41(1):110–118. doi: 10.1128/jvi.41.1.110-118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., McFarland H., McFarlin D. Protein A-peroxidase: a valluable tool for the localization of antigens. J Histochem Cytochem. 1977 Nov;25(11):1201–1206. doi: 10.1177/25.11.199666. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Ernst H., Shatkin A. J. Reovirus hemagglutinin mRNA codes for two polypeptides in overlapping reading frames. Proc Natl Acad Sci U S A. 1985 Jan;82(1):48–52. doi: 10.1073/pnas.82.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Fujimura T., Wickner R. B. Site-specific binding of viral plus single-stranded RNA to replicase-containing open virus-like particles of yeast. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4411–4415. doi: 10.1073/pnas.85.12.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. J., Bobek L. A., Brennan V. E., Reilly J. D., Bruenn J. A. There are at least two yeast viral double-stranded RNAs of the same size: an explanation for viral exclusion. Cell. 1982 Nov;31(1):193–200. doi: 10.1016/0092-8674(82)90419-6. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Fry C. M., Rowlands D. J., Bittle J. L., Houghten R. A., Lerner R. A., Brown F. Immune response to uncoupled peptides of foot-and-mouth disease virus. Immunology. 1987 May;61(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Esteban R., Wickner R. B. In vitro L-A double-stranded RNA synthesis in virus-like particles from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4433–4437. doi: 10.1073/pnas.83.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Wickner R. B. Gene overlap results in a viral protein having an RNA binding domain and a major coat protein domain. Cell. 1988 Nov 18;55(4):663–671. doi: 10.1016/0092-8674(88)90225-5. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V. Birnavirus RNA polymerase is related to polymerases of positive strand RNA viruses. Nucleic Acids Res. 1988 Aug 11;16(15):7735–7735. doi: 10.1093/nar/16.15.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Brennan V., Bruenn J. A. No homology between double-stranded RNA and nuclear DNA of yeast. J Virol. 1978 Dec;28(3):1002–1005. doi: 10.1128/jvi.28.3.1002-1005.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W. A., West K., Gallagher J. F. Importance of initiation factor preparations in the translation of reovirus and globin mRNAs lacking a 5'-terminal 7-methylguanosine. J Biol Chem. 1977 Dec 10;252(23):8489–8497. [PubMed] [Google Scholar]
- Holm C. A., Oliver S. G., Newman A. M., Holland L. E., McLaughlin C. S., Wagner E. K., Warner R. C. The molecular weight of yeast P1 double-stranded RNA. J Biol Chem. 1978 Nov 25;253(22):8332–8336. [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Translational initiation factor and ribosome association with the cytoskeletal framework fraction from HeLa cells. Cell. 1984 May;37(1):85–93. doi: 10.1016/0092-8674(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Jacks T., Townsley K., Varmus H. E., Majors J. Two efficient ribosomal frameshifting events are required for synthesis of mouse mammary tumor virus gag-related polyproteins. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4298–4302. doi: 10.1073/pnas.84.12.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Varmus H. E. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science. 1985 Dec 13;230(4731):1237–1242. doi: 10.1126/science.2416054. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S. M., Atwater J. A., Samuel C. E. Biosynthesis of reovirus-specified polypeptides. Molecular cDNA cloning and nucleotide sequence of the reovirus serotype 1 Lang strain bicistronic s1 mRNA which encodes the minor capsid polypeptide sigma 1a and the nonstructural polypeptide sigma 1bNS. Biochem Biophys Res Commun. 1986 Oct 30;140(2):508–514. doi: 10.1016/0006-291x(86)90761-8. [DOI] [PubMed] [Google Scholar]
- Nemeroff M. E., Bruenn J. A. Conservative replication and transcription of Saccharomyces cerevisiae viral double-stranded RNA in vitro. J Virol. 1986 Mar;57(3):754–758. doi: 10.1128/jvi.57.3.754-758.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff M. E., Pietras D. F., Bruenn J. A. Construction of full-length cDNA copies of viral double-stranded RNA. Virus Genes. 1988 Jun;1(3):243–253. doi: 10.1007/BF00572703. [DOI] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. D., Bruenn J., Held W. The capsid polypeptides of the yeast viruses. Biochem Biophys Res Commun. 1984 Jun 15;121(2):619–625. doi: 10.1016/0006-291x(84)90227-4. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz P., Stucka R., Feldmann H., Combriato G., Klobeck H. G., Fittler F. Sequence of a cDNA clone encompassing the complete mature human prostate specific antigen (PSA) and an unspliced leader sequence. Nucleic Acids Res. 1988 Jul 11;16(13):6226–6226. doi: 10.1093/nar/16.13.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Yeast L dsRNA consists of at least three distinct RNAs; evidence that the non-Mendelian genes [HOK], [NEX] and [EXL] are on one of these dsRNAs. Cell. 1982 Dec;31(2 Pt 1):429–441. doi: 10.1016/0092-8674(82)90136-2. [DOI] [PubMed] [Google Scholar]
- Tarlow O., McCorquodale J. G., McCrae M. A. Molecular cloning and sequencing of the gene (M2) encoding the major virion structural protein (mu 1-mu 1C) of serotypes 1 and 3 of mammalian reovirus. Virology. 1988 May;164(1):141–146. doi: 10.1016/0042-6822(88)90629-0. [DOI] [PubMed] [Google Scholar]
- Thiele D. J., Hannig E. M., Leibowitz M. J. Multiple L double-stranded RNA species of Saccharomyces cerevisiae: evidence for separate encapsidation. Mol Cell Biol. 1984 Jan;4(1):92–100. doi: 10.1128/mcb.4.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M., Lamb R. A., Paterson R. G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988 Sep 9;54(6):891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. Induction of yeast killer factor mutations. J Bacteriol. 1977 Oct;132(1):346–348. doi: 10.1128/jb.132.1.346-348.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded RNA replication in yeast: the killer system. Annu Rev Biochem. 1986;55:373–395. doi: 10.1146/annurev.bi.55.070186.002105. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., Joklik W. K. Evolution of reovirus genes: a comparison of serotype 1, 2, and 3 M2 genome segments, which encode the major structural capsid protein mu 1C. Virology. 1988 Apr;163(2):603–613. doi: 10.1016/0042-6822(88)90301-7. [DOI] [PubMed] [Google Scholar]
- Williams T. L., Leibowitz M. J. Conservative mechanism of the in vitro transcription of killer virus of yeast. Virology. 1987 May;158(1):231–234. doi: 10.1016/0042-6822(87)90258-3. [DOI] [PubMed] [Google Scholar]
- Wilson W., Braddock M., Adams S. E., Rathjen P. D., Kingsman S. M., Kingsman A. J. HIV expression strategies: ribosomal frameshifting is directed by a short sequence in both mammalian and yeast systems. Cell. 1988 Dec 23;55(6):1159–1169. doi: 10.1016/0092-8674(88)90260-7. [DOI] [PubMed] [Google Scholar]
- el-Sherbeini M., Bostian K. A. Viruses in fungi: infection of yeast with the K1 and K2 killer viruses. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4293–4297. doi: 10.1073/pnas.84.12.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]