Abstract
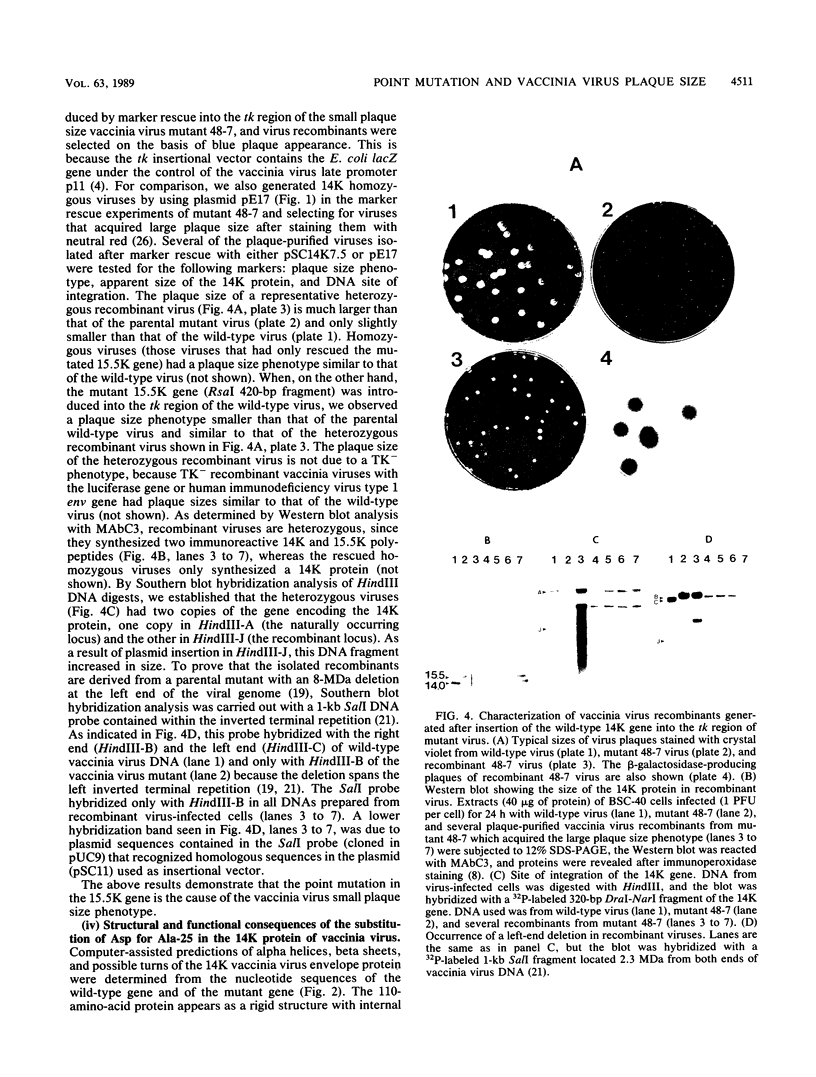
The molecular defect responsible for a structural and functional abnormality of the 14,000-molecular-weight (14K) envelope protein of vaccinia virus has been identified. Through DNA sequence analysis of the entire 14K gene from wild-type vaccinia virus and three vaccinia virus mutants, a single base change of C to A was found that resulted in the substitution of Asp for Ala-25. This mutation is responsible for protein size abnormality, as documented by cell-free translation in a rabbit reticulocyte lysate of in vitro mRNA transcripts. In addition, through marker rescue experiments we show that this mutation is responsible for the small plaque size phenotype of vaccinia virus mutants. The structural consequence of the point mutation is a possible turn in an alpha-helix domain with destabilization of a hydrophobic interaction at the N terminus, resulting in monomers and trimers of vaccinia virus 14K protein with decreased electrophoretic mobilities. The functional consequence of the point mutation is a reduction in virulence of the virus.
Full text
PDF

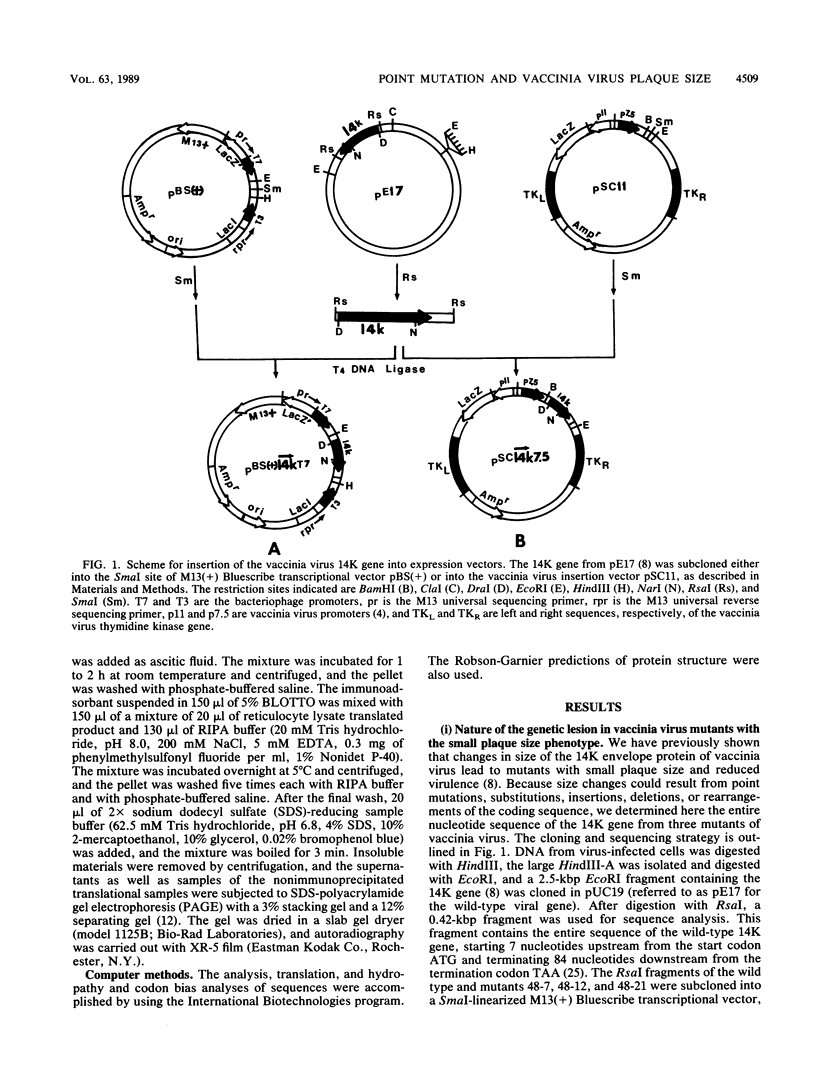
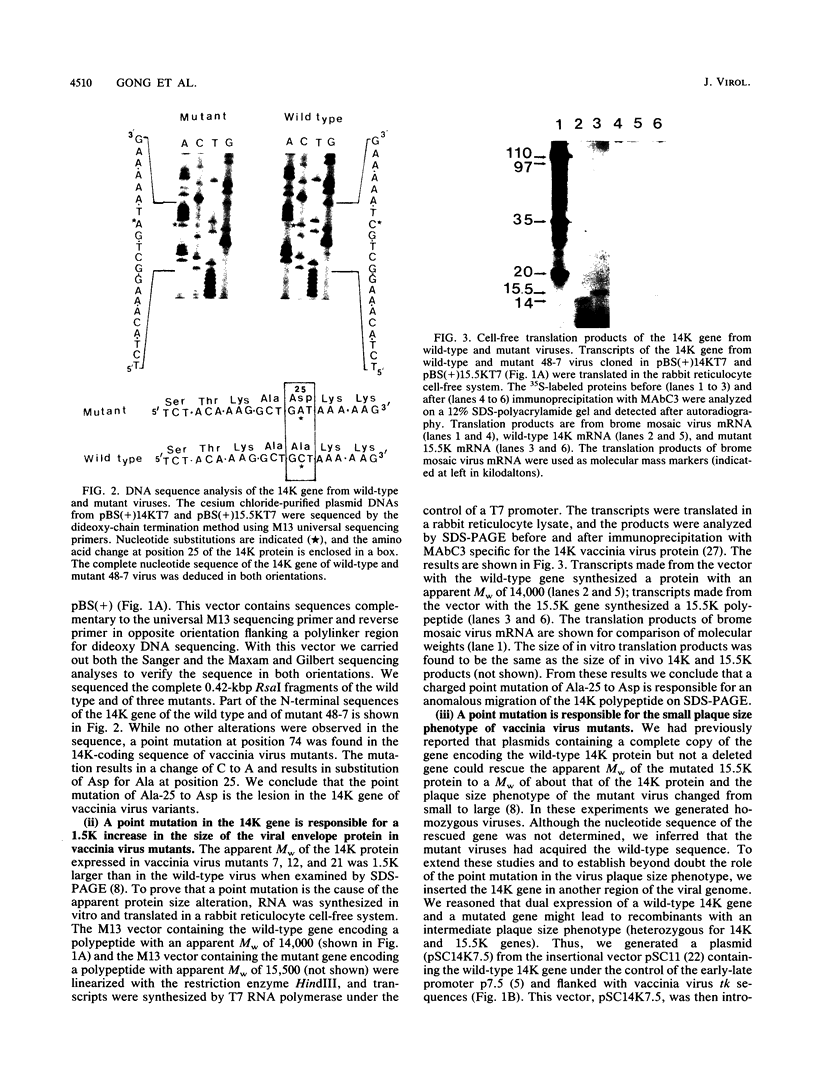



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown F., Schild G. C., Ada G. L. Recombinant vaccinia viruses as vaccines. Nature. 1986 Feb 13;319(6054):549–550. doi: 10.1038/319549a0. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Puckett C., Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985 Apr;54(1):30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S., Esteban M. Isolation and characterization of attenuated mutants of vaccinia virus. Virology. 1987 Aug;159(2):408–422. doi: 10.1016/0042-6822(87)90480-6. [DOI] [PubMed] [Google Scholar]
- Dallo S., Rodriguez J. F., Esteban M. A 14K envelope protein of vaccinia virus with an important role in virus-host cell interactions is altered during virus persistence and determines the plaque size phenotype of the virus. Virology. 1987 Aug;159(2):423–432. doi: 10.1016/0042-6822(87)90481-8. [DOI] [PubMed] [Google Scholar]
- Flexner C., Hügin A., Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987 Nov 19;330(6145):259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Evelegh C. M., Cunniff N. F., Skiadopoulos M. H., Floroff M. R., Denman J. E., Bayley S. T. Use of deletion and point mutants spanning the coding region of the adenovirus 5 E1A gene to define a domain that is essential for transcriptional activation. Virology. 1988 Apr;163(2):494–502. doi: 10.1016/0042-6822(88)90290-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Paez E., Dallo S., Esteban M. Generation of a dominant 8-MDa deletion at the left terminus of vaccinia virus DNA. Proc Natl Acad Sci U S A. 1985 May;82(10):3365–3369. doi: 10.1073/pnas.82.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez E., Dallo S., Esteban M. Virus attenuation and identification of structural proteins of vaccinia virus that are selectively modified during virus persistence. J Virol. 1987 Aug;61(8):2642–2647. doi: 10.1128/jvi.61.8.2642-2647.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez E., Esteban M. Stability of vaccinia virus DNA during persistent infections: accumulation of left-end deletions and of tandem repeats at both ends of the viral genome and prevention by interferon. Virology. 1988 Mar;163(1):145–154. doi: 10.1016/0042-6822(88)90241-3. [DOI] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Naeve C. W. The fusion glycoprotein of Sendai virus: sequence analysis of an epitope involved in fusion and virus neutralization. Virology. 1987 Apr;157(2):556–559. doi: 10.1016/0042-6822(87)90301-1. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Andrew M. E., Phillips S. M., Boyle D. B., Coupar B. E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987 Oct 8;329(6139):545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- Redfield R. R., Wright D. C., James W. D., Jones T. S., Brown C., Burke D. S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987 Mar 12;316(11):673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- Rodriguez D., Rodriguez J. R., Rodriguez J. F., Trauber D., Esteban M. Highly attenuated vaccinia virus mutants for the generation of safe recombinant viruses. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1287–1291. doi: 10.1073/pnas.86.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Esteban M. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. J Virol. 1987 Nov;61(11):3550–3554. doi: 10.1128/jvi.61.11.3550-3554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Esteban M. Plaque size phenotype as a selectable marker to generate vaccinia virus recombinants. J Virol. 1989 Feb;63(2):997–1001. doi: 10.1128/jvi.63.2.997-1001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Janeczko R., Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985 Nov;56(2):482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Paez E., Esteban M. A 14,000-Mr envelope protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987 Feb;61(2):395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Kawaoka Y., Bean W. J., Jr Molecular changes in A/Chicken/Pennsylvania/83 (H5N2) influenza virus associated with acquisition of virulence. Virology. 1986 Mar;149(2):165–173. doi: 10.1016/0042-6822(86)90118-2. [DOI] [PubMed] [Google Scholar]
- Welham M. J., Wyke J. A. A single point mutation has pleiotropic effects on pp60v-src function. J Virol. 1988 Jun;62(6):1898–1906. doi: 10.1128/jvi.62.6.1898-1906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]