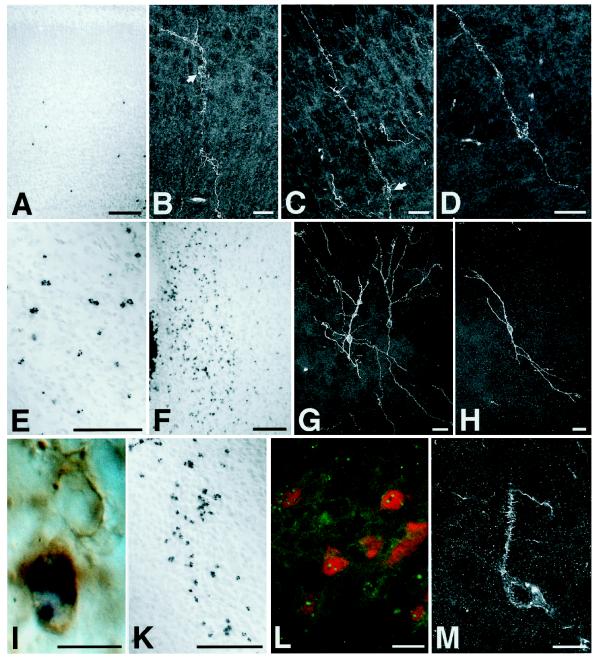
Figure 2.
ES-cell-derived neural precursors injected into the telencephalic vesicle of fetal rats incorporate individually into a variety of host brain regions and differentiate into neurons. Donor cells are identified by in situ hybridization using a digoxigenin-labeled probe to mouse satellite DNA (A, E, F, and I–L). Immunofluorescence detection of the mouse-specific antigen M6 and confocal laser microscopy were used to reconstruct individual neuronal profiles (B–D, G, H, and M). (A–D) Six days after injection into the telencephalic vesicle of an E17 rat, donor cells have left the ventricle and incorporated into the host cortex. The ES-cell-derived neurons show prominent apical dendrites and basal axons entering the corpus callosum, a morphology appropriate for cortical projection neurons (arrows: perikaryon). Note the characteristic pyramidal morphology in D. (E) Incorporated donor cells in the striatum of a 2-week-old rat transplanted at E18. (F–H) Donor-derived cells in the host hypothalamus. In contrast to cortex, neurons incorporating into the diencephalon frequently exhibited multipolar morphologies (G and H). (I) Host- and donor-derived neurons in the septum of a newborn rat. Both neurons show expression of microtubule-associated protein 2; the donor-derived cell is identified by in situ hybridization. (K–M) Incorporated cells in the host thalamus of newborn (K) and 2-week-old rats (L and M). In L, ES-cell-derived neurons are visualized by fluorescence in situ hybridization (green dots) and subsequent immunofluorescence analysis with an antibody to the nuclear neuronal antigen NeuN (red). Note the mature neuronal phenotype of the integrated cells with the presence of dendritic spines (M). [Bars = 100 μm (A, E, F, and K), 20 μm (B–D, G, H, L, and M), and 10 μm (I).]