Abstract
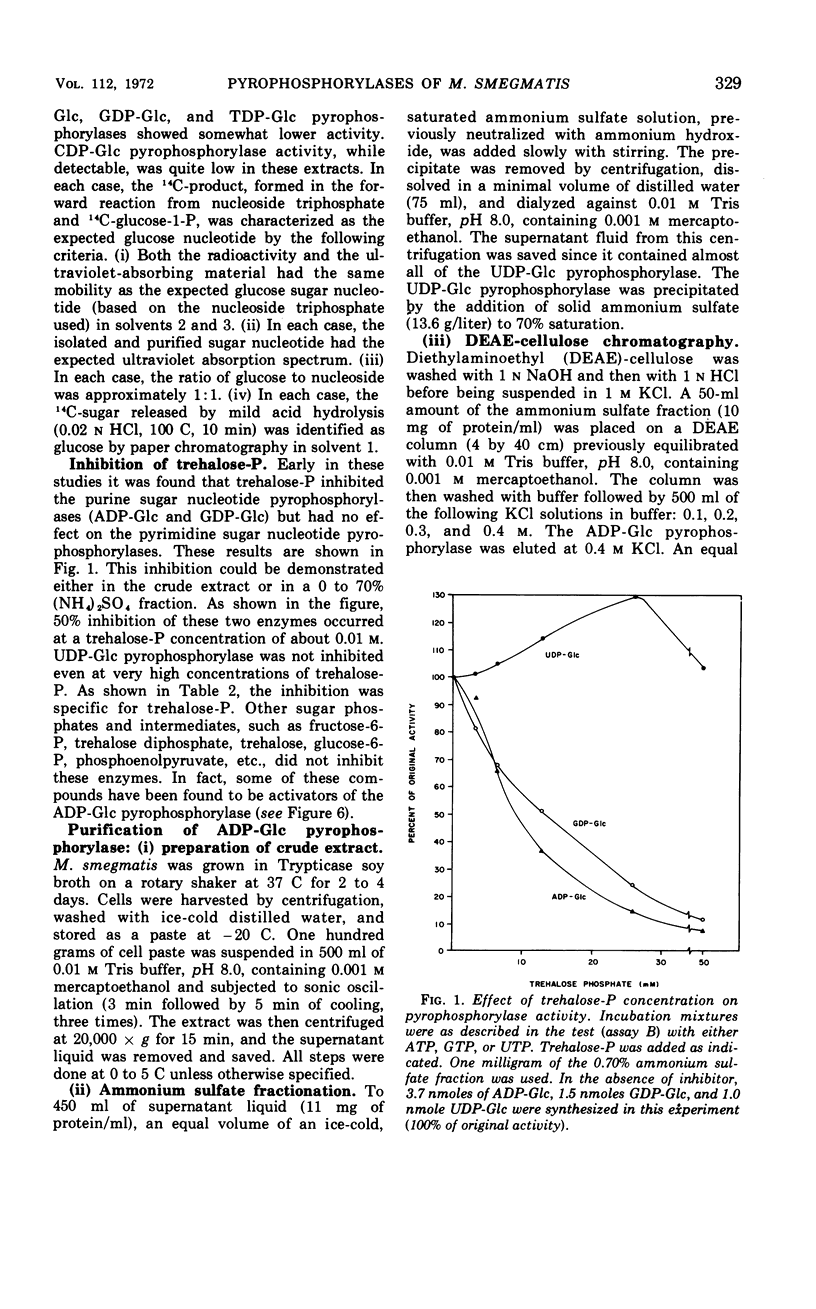
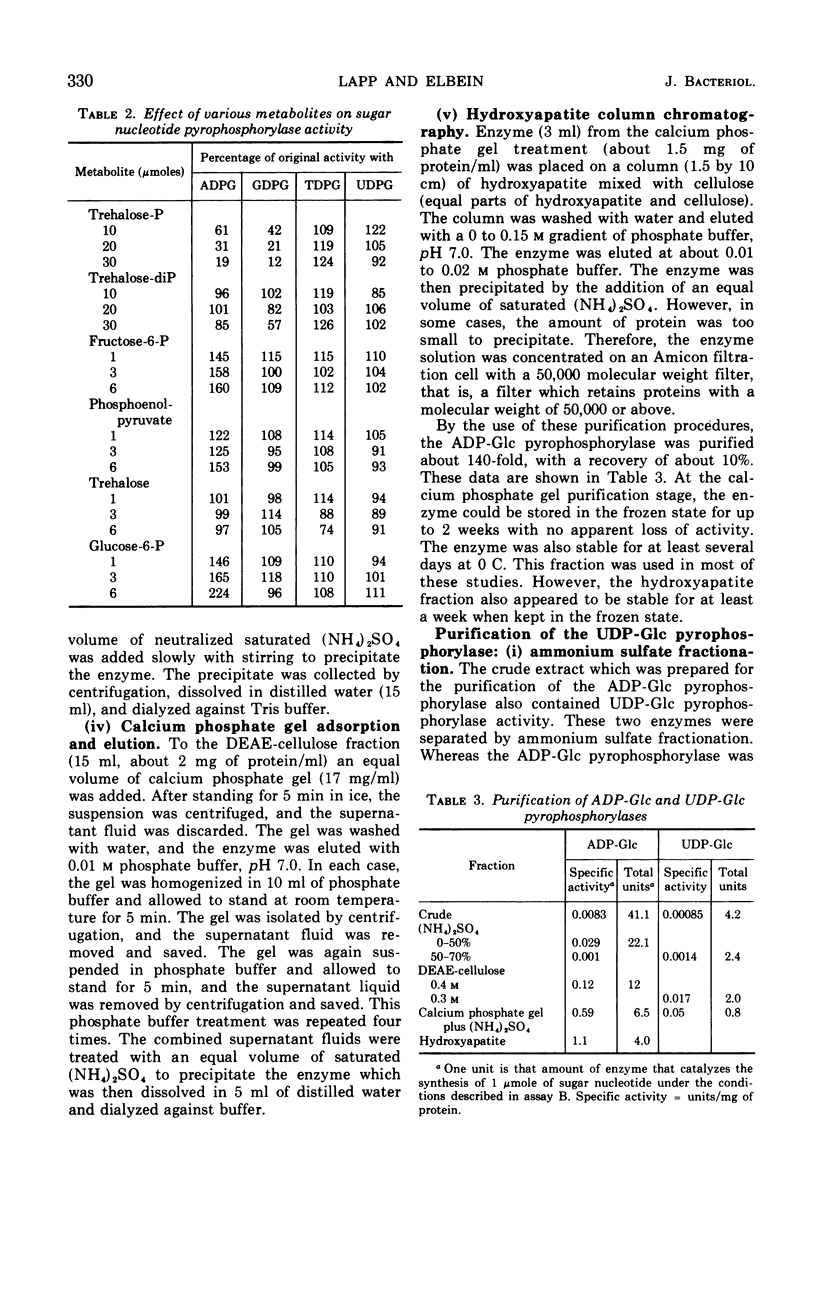
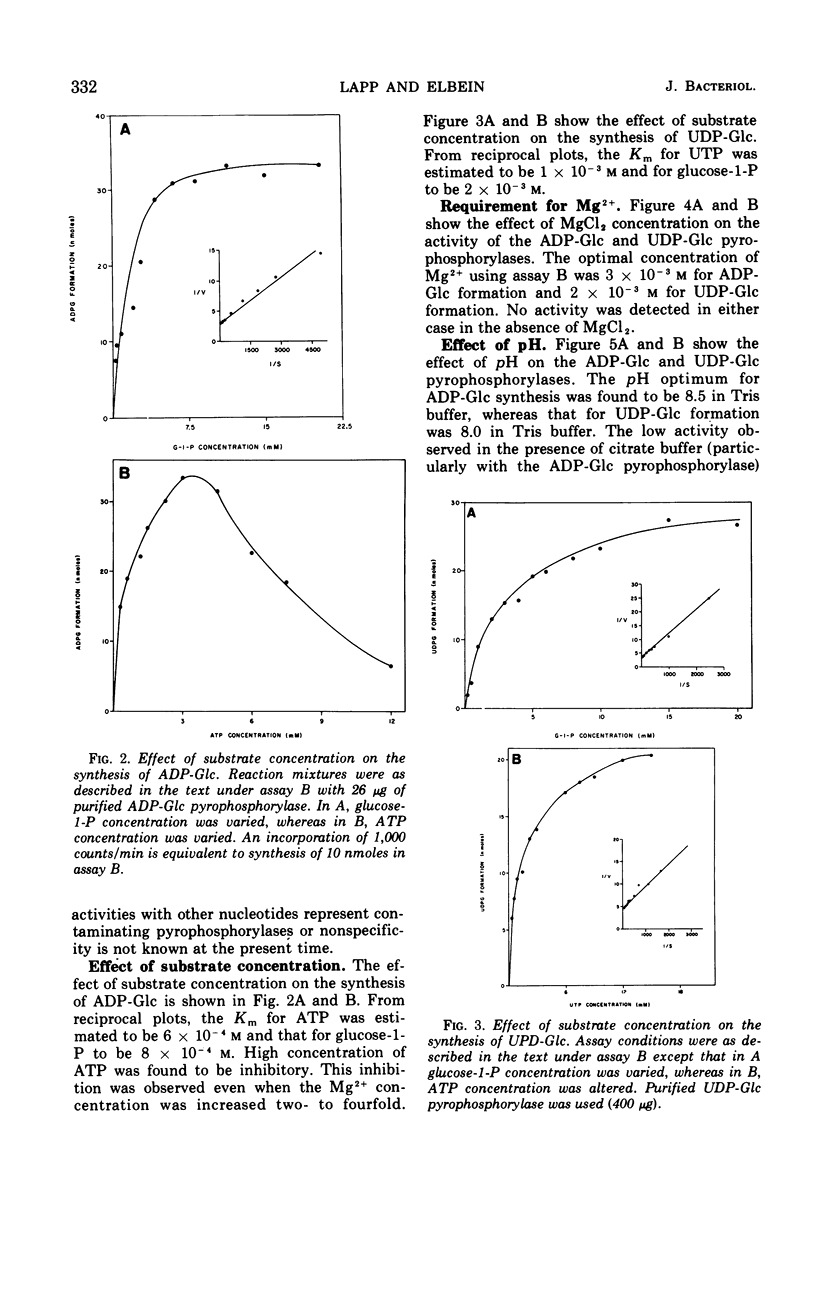
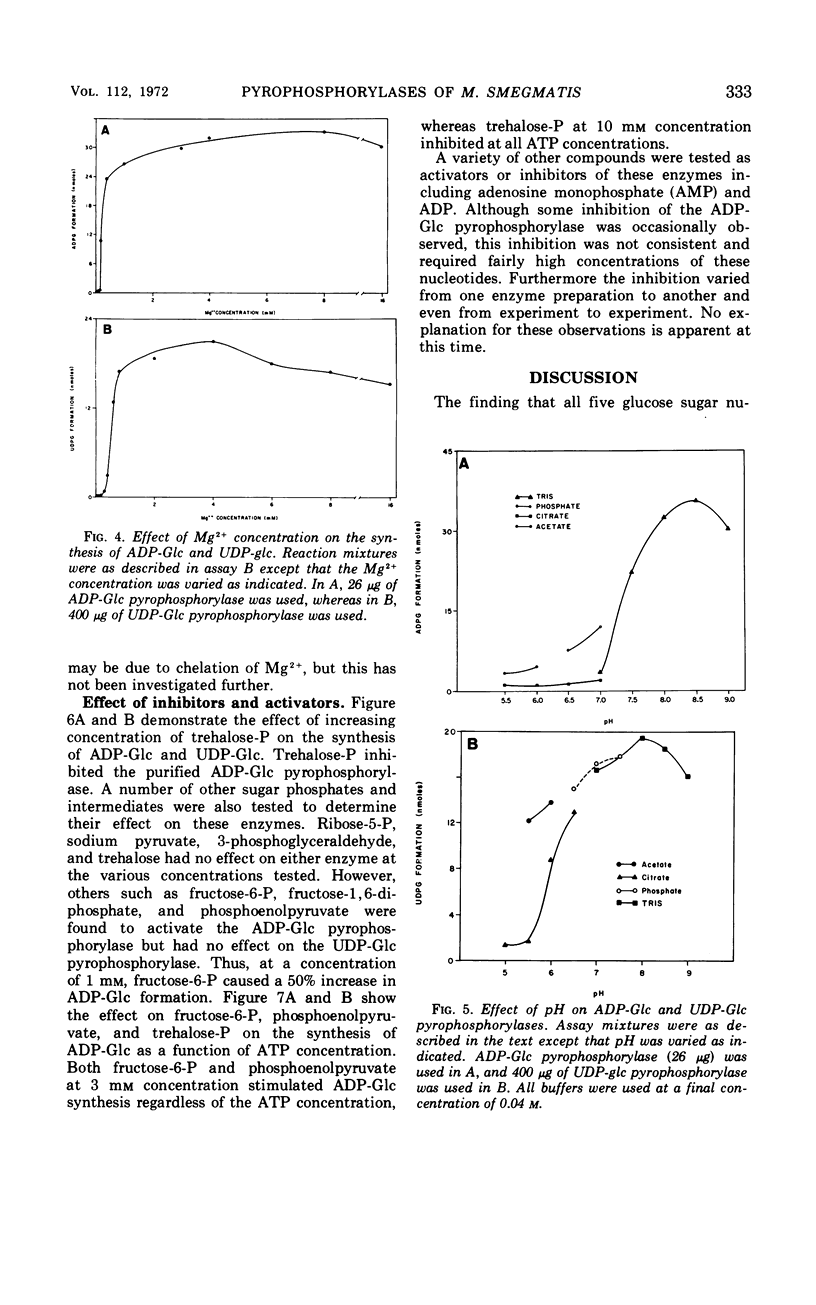
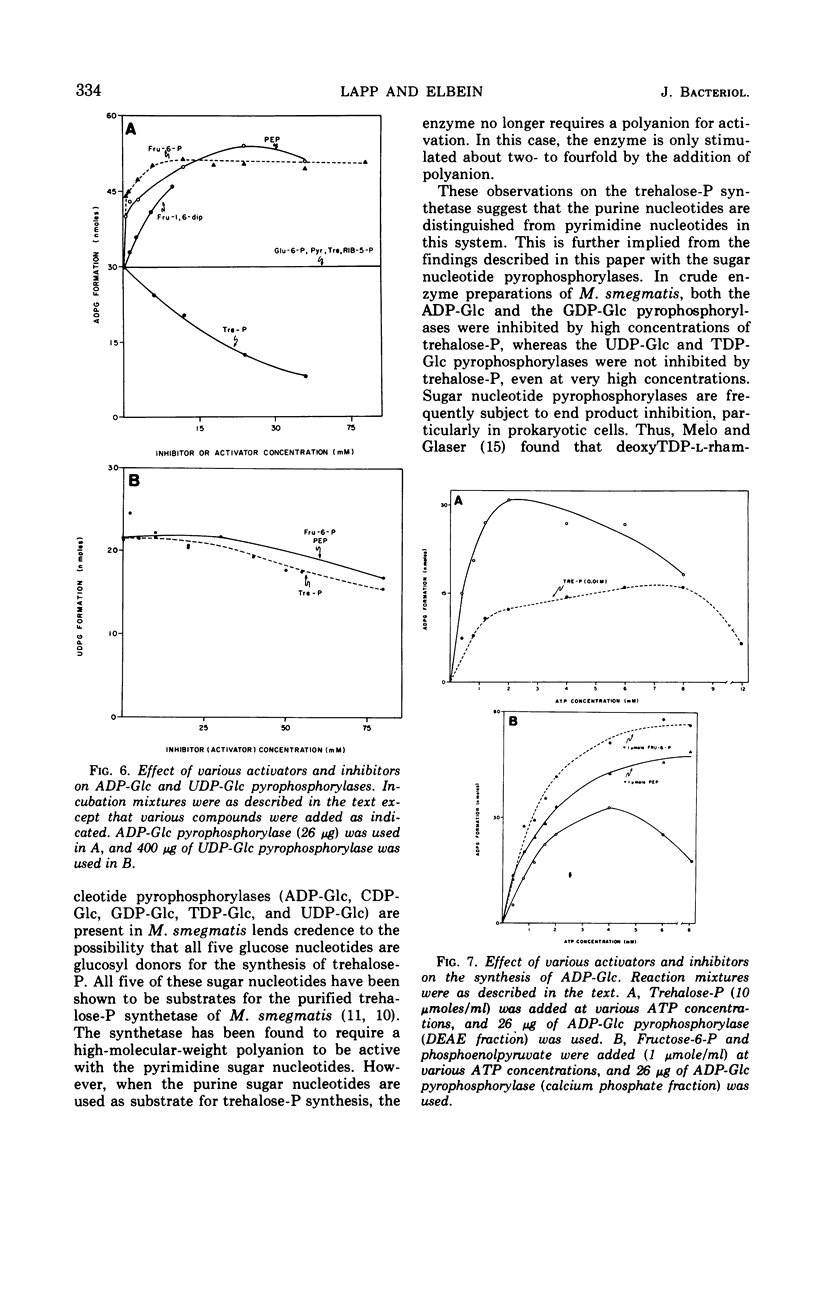
Crude extracts of Mycobacterium smegmatis catalyzed the synthesis of adenosine diphosphate-glucose (ADP-Glc), cytidine diphosphate-glucose, guanosine diphosphate-glucose (GDP-Glc), thymidine diphosphate-glucose (TDP-Glc), and uridine diphosphate-glucose (UDP-Glc). In these crude enzyme fractions, high concentrations of trehalose-P inhibited the ADP-Glc and GDP-Glc pyrophosphorylases but did not effect the UDP-Glc or TDP-Glc pyrophosphorylases. Both the ADP-Glc pyrophosphorylase and the UDP-Glc pyrophosphorylase were partially purified (about 140-fold and 60-fold, respectively), and their properties were compared. For the ADP-Glc pyrophosphorylase, the Km for adenosine triphosphate was 6 × 10−4m, whereas that for glucose-1-P was 8 × 10−4m. The optimal concentration of Mg2+ was 1 × 10−3m, and the pH optimum was 8.5. For the UDP-Glc pyrophosphorylase, the Km for uridine triphosphate was 1 × 10−3m and for glucose-1-P was 2 × 10−3m. The optimal Mg2+ concentration was 1 × 10−3m, and the pH optimum was about 8.0. The purified ADP-Glc pyrophosphorylase was inhibited by fructose-6-P, fructose-1, 6-diphosphate, glucose-6-P, and phosphoenolpyruvate. On the other hand, trehalose, trehalose diphosphate, sodium pyruvate, and ribose-5-P did not effect the ADP-Glc pyrophosphorylase. None of these compounds, including trehalose-P, had any effect on the UDP-Glc pyrophosphorylase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht G. J., Bass S. T., Seifert L. L., Hansen R. G. Crystallization and properties of uridine diphosphate glucose pyrophosphorylase from liver. J Biol Chem. 1966 Jun 25;241(12):2968–2975. [PubMed] [Google Scholar]
- Antoine A. D., Tepper B. S. Characterization of glycogens from mycobacteria. Arch Biochem Biophys. 1969 Oct;134(1):207–213. doi: 10.1016/0003-9861(69)90267-7. [DOI] [PubMed] [Google Scholar]
- Antoine A. D., Tepper B. S. Environmental control of glycogen and lipid content of Mycobacterium phlei. J Gen Microbiol. 1969 Feb;55(2):217–226. doi: 10.1099/00221287-55-2-217. [DOI] [PubMed] [Google Scholar]
- BERNSTEIN R. L., ROBBINS P. W. CONTROL ASPECTS OF URIDINE 5'-DIPHOSPHATE GLUCOSE AND THYMIDINE 5'-DIPHOSPHATE GLUCOSE SYNTHESIS BY MICROBIAL ENZYMES. J Biol Chem. 1965 Jan;240:391–397. [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Kornfeld R. H., Ginsburg V. Control of synthesis of guanosine 5'-diphosphate D-mannose and guanosine 5'-diphosphate L-fucose in bacteria. Biochim Biophys Acta. 1966 Mar 28;117(1):79–87. doi: 10.1016/0304-4165(66)90154-1. [DOI] [PubMed] [Google Scholar]
- Liu C., Patterson B. W., Lapp D., Elbein A. D. Trehalose phosphate synthesis from uridine diphosphate glucose or guanosine diphosphate glucose. Activation of uridine diphosphate-glucose: trehalose phosphate synthetase by polynucleotides. J Biol Chem. 1969 Jul 10;244(13):3728–3731. [PubMed] [Google Scholar]
- MACDONALD D. L., WONG R. Y. A CHEMICAL SYNTHESIS OF TREHALOSE 6-PHOSPHATE. Biochim Biophys Acta. 1964 May 11;86:390–392. doi: 10.1016/0304-4165(64)90066-2. [DOI] [PubMed] [Google Scholar]
- MAYER R. M., GINSBURG V. PURIFICATION AND PROPERTIES OF CYTIDINE DIPHOSPHATE D-GLUCOSE PYROPHOSPHORYLASE FROM SALMONELLA PARATYPHI A. J Biol Chem. 1965 May;240:1900–1904. [PubMed] [Google Scholar]
- MELO A., GLASER L. THE NUCLEOTIDE SPECIFICITY AND FEEDBACK CONTROL OF THYMIDINE DIPHOSPHATE D-GLUCOSE PYROPHOSPHORYLASE. J Biol Chem. 1965 Jan;240:398–405. [PubMed] [Google Scholar]
- Matula M., Mitchell M., Elbein A. D. Partial purification and properties of a highly specific trehalose phosphate phosphatase from Mycobacterium smegmatis. J Bacteriol. 1971 Jul;107(1):217–222. doi: 10.1128/jb.107.1.217-222.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Multiple molecular forms of uridine diphosphate glucose pyrophosphorylase from Salmonella typhimurium. I. Catalytic properties of various forms. J Biol Chem. 1971 Jul 25;246(14):4386–4396. [PubMed] [Google Scholar]
- SHEN L., PREISS J. BIOSYNTHESIS OF BACTERIAL GLYCOGEN. I. PURIFICATION AND PROPERTIES OF THE ADENOSINE DIPHOSPHOGLUCOSE PYROPHOSPHORYLASE OF ARTHROBACTER SPECIES NRRL B1973. J Biol Chem. 1965 Jun;240:2334–2340. [PubMed] [Google Scholar]
- Sanwal G. G., Preiss J. Biosynthesis of starch in Chlorella pyrenoidosa. II. Regulation of ATP: alpha-D-glucose 1-phosphate adenyl transferase (ADP-glucose pyrophosphorylase) by inorganic phosphate and 3-phosphoglycerate. Arch Biochem Biophys. 1967 Mar;119(1):454–469. doi: 10.1016/0003-9861(67)90477-8. [DOI] [PubMed] [Google Scholar]
- Shen L. C., Atkinson D. E. Regulation of adenosine diphosphate glucose synthase from Escherichia coli. Interactions of adenylate energy charge and modifier concentrations. J Biol Chem. 1970 Aug 10;245(15):3996–4000. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Fukunaga K., Petricciani J. C. Purification and specific kinetic properties of erythrocyte uridine diphosphate glucose pyrophosphorylase. J Biol Chem. 1969 Feb 10;244(3):1008–1015. [PubMed] [Google Scholar]
- VILLAR-PALASI C., LARNER J. Uridinediphosphate glucose pyrophosphorylase from skeletal muscle. Arch Biochem Biophys. 1960 Jan;86:61–66. doi: 10.1016/0003-9861(60)90368-4. [DOI] [PubMed] [Google Scholar]
- WINDER F., BRENNAN P. THE ACCUMULATION OF FREE TREHALOSE BY MYCOBACTERIA EXPOSED TO ISONIAZID. Biochim Biophys Acta. 1964 Aug 19;90:442–444. doi: 10.1016/0304-4165(64)90222-3. [DOI] [PubMed] [Google Scholar]
- Winder F. G., Rooney S. A. The effects of isoniazid on the carbohydrates of Mycobacterium tuberculosis BCG. Biochem J. 1970 Apr;117(2):355–368. doi: 10.1042/bj1170355. [DOI] [PMC free article] [PubMed] [Google Scholar]


