Abstract
A mutant F′ plasmid has been isolated in a strain of Salmonella typhimurium harboring Fts114lac. This mutant, designated FlacS, exhibits unique genetic stability in strains of S. typhimurium and Escherichia coli. It shows no thermolability and is lost at frequencies of 20 to 100 times less than the wild-type F′lac (F42) in the same genetic backgrounds. The FlacS is also insensitive to conventional plasmid curing agents, whereas both Fts114lac and F42 are readily cured. The nature of the mutation(s) conferring stability to the FlacS is unclear, but plasmid linkage has been established. The high frequency of conjugal transfer of the FlacS and its behavior in recombination-deficient strains of S. typhimurium and E. coli argue against its stability being due to stable chromosomal integration. The FlacS is also capable of transferring chromosomal markers in S. typhimurium and E. coli mating systems. No major differences in chromosomal mobilization have been observed among F42, Fts114lac, and FlacS donors of either genus.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron L. S., Gemski P., Johnson E. M., Wohlhieter J. A. Intergeneric bacterial matings. Bacteriol Rev. 1968 Dec;32(4 Pt 1):362–369. [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R., Epstein W. Transposition of the Lac region of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:393–401. doi: 10.1101/sqb.1966.031.01.051. [DOI] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume A. J., Balbinder E. The tryptophan operon of Salmonella typhimurium. Fine structure analysis by deletion mapping and abortive transduction. Genetics. 1966 Mar;53(3):577–592. doi: 10.1093/genetics/53.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Levine M. Intermediates in the synthesis of phage P22 DNA. Cold Spring Harb Symp Quant Biol. 1968;33:659–667. doi: 10.1101/sqb.1968.033.01.075. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Boyer H. W. DNA restriction and modification mechanisms in bacteria. Annu Rev Microbiol. 1971;25:153–176. doi: 10.1146/annurev.mi.25.100171.001101. [DOI] [PubMed] [Google Scholar]
- Calvo J. M., Worden H. E. A multisite-mutation map of the leucine operon of Salmonella typhimurium. Genetics. 1970 Feb;64(2):199–214. doi: 10.1093/genetics/64.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C., Moody E. E. Chromosomal transfer from "recombination-deficient" strains of Escherichia coli K-12. Genetics. 1966 Apr;53(4):717–726. doi: 10.1093/genetics/53.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson A. M., Colson C., Van Pel A. Host-controlled restriction mutants of Salmonella typhimurium. J Gen Microbiol. 1969 Sep;58(1):57–64. doi: 10.1099/00221287-58-1-57. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Bacterial conjugation. Annu Rev Microbiol. 1969;23:69–136. doi: 10.1146/annurev.mi.23.100169.000441. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Renshaw J. F+ strains of Escherichia coli K-12 defective in Hfr formation. Genetics. 1969 Sep;63(1):7–26. doi: 10.1093/genetics/63.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Mutations de l'épisome F d'Escherichia coli K 12. II. Mutants à réplication thermosensible. Ann Inst Pasteur (Paris) 1967 Apr;112(4):397–418. [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S K, Adelberg E A. The Occurrence of a Genetic Transposition in a Strain of Escherichia Coli. Genetics. 1962 May;47(5):577–585. doi: 10.1093/genetics/47.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earhart C. F. The association of host and phage DNA with the membrane of Escherichia coli. Virology. 1970 Oct;42(2):420–436. [PubMed] [Google Scholar]
- Eggertsson G., Adelberg E. A. Map positions and specificities of suppressor mutations in Escherichia coli K-12. Genetics. 1965 Aug;52(2):319–340. doi: 10.1093/genetics/52.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstark A., Eisenstark R., van Dillewijn J., Rörsch A. Radiation--sensitive and recombinationless mutants of Salmonella typhimurium. Mutat Res. 1969 Nov-Dec;8(3):497–504. doi: 10.1016/0027-5107(69)90066-9. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Hanawalt P. Isolation and characterization of the DNA replication complex from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):301–322. doi: 10.1016/0022-2836(70)90032-x. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., ADELBERG E. A. Transfert de caractéres génétiques par incorporation au facteur sexuel d'Escherichia coli. C R Hebd Seances Acad Sci. 1959 Jul 6;249(1):189–191. [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Kétyi I. Isolation of Hfr derivative by the use of Shigella flexneri 4b-modified F factor. Acta Microbiol Acad Sci Hung. 1970;17(2):167–173. [PubMed] [Google Scholar]
- Levy S. B. Physical and functional characteristics of R-factor deoxyribonucleic acid segregated into Escherichia coli minicells. J Bacteriol. 1971 Oct;108(1):300–308. doi: 10.1128/jb.108.1.300-308.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Malamy M. H. Studies with bacteriophage phi II. Events following infection of male and female derivatives of Escherichia coli K-12. J Virol. 1970 Jan;5(1):72–78. doi: 10.1128/jvi.5.1.72-78.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKELA P. H., LEDERBERG J., LEDERBERG E. M. Patterns of sexual recombination in enteric bacteria. Genetics. 1962 Oct;47:1427–1439. doi: 10.1093/genetics/47.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby L. R., Barlow G. H., Doi R. H., Jacob M., Spiegelman S. Comparison of two serologically distinct ribonucleic acid bacteriophages. I. Properties of the viral particles. J Bacteriol. 1966 Jan;91(1):442–448. doi: 10.1128/jb.91.1.442-448.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., RAMAKRISHNAN T. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI. IV. EFFECT OF A CHROMOSOMAL DELETION ON CHROMOSOME TRANSFER. J Bacteriol. 1964 Aug;88:367–373. doi: 10.1128/jb.88.2.367-373.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salivar W. O., Sinsheimer R. L. Intracellular location and number of replicating parental DNA molecules of bacteriophages lambda and phi-X174. J Mol Biol. 1969 Apr 14;41(1):39–65. doi: 10.1016/0022-2836(69)90124-7. [DOI] [PubMed] [Google Scholar]
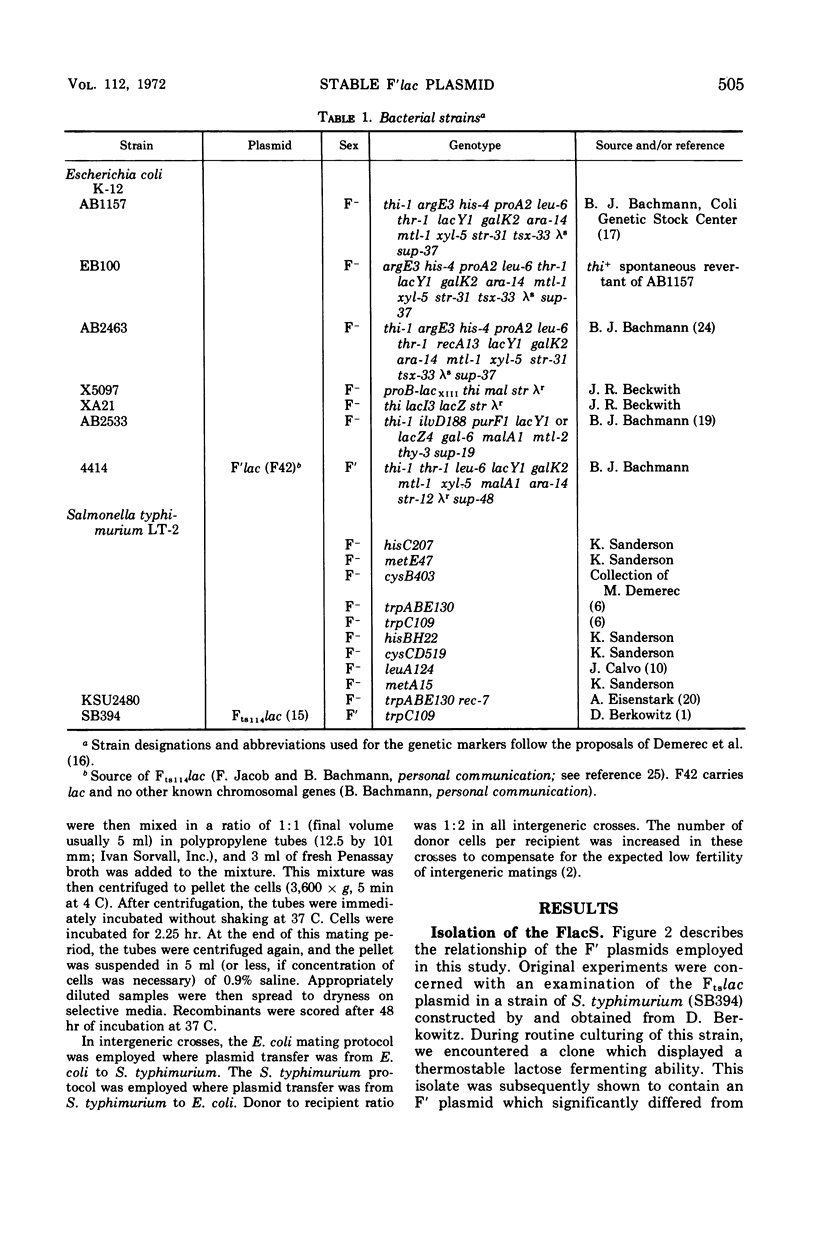
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Properties of the growing point region in the bacterial chromosome. Biochim Biophys Acta. 1967 Dec 19;149(2):519–531. doi: 10.1016/0005-2787(67)90180-3. [DOI] [PubMed] [Google Scholar]
- Stadler J., Adelberg E. A. Temperature dependence of sex-factor maintenance in Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):447–449. doi: 10.1128/jb.109.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Membrane attachment of replicating parental DNA molecules of bacteriophage M13. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1035–1041. doi: 10.1016/0006-291x(71)90008-8. [DOI] [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Kubo N., Nakamura S. Effective elimination of drug resistance and sex factors in Escherichia coli by sodium dodecyl sulfate. J Bacteriol. 1968 Mar;95(3):1078–1089. doi: 10.1128/jb.95.3.1078-1089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Chromosomal mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jan 28;63(2):281–294. doi: 10.1016/0022-2836(72)90375-0. [DOI] [PubMed] [Google Scholar]


