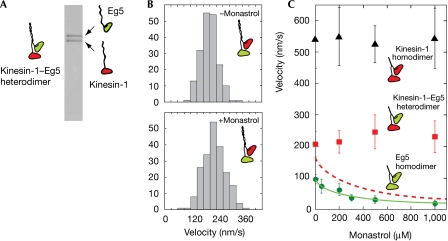
Figure 2.
Microtubule sliding by kinesin-1–Eg5 heterodimers. (A) SDS–polyacrylamide gel electrophoresis of kinesin-1–Eg5 heterodimers. Two heavy chains are present in equal amounts. (B) Histograms of MT sliding velocities driven by kinesin-1–Eg5 heterodimer in the absence (top) and presence (bottom) of 1 mM monastrol. (C) MT sliding velocities driven by kinesin-1–Eg5 heterodimer (squares), kinesin-1 homodimer (triangles) and Eg5 homodimer (circles). Monastrol inhibited MT sliding by the Eg5 homodimer. A fit (solid line) to a hyperbolic inhibition equation V=Intcpt−(amplitude × [monastrol]/(K50%+[monastrol])) indicated that 50% inhibition occurs at 256 μM monastrol. The predicted velocity of kinesin-1–Eg5 heterodimer on a model with strict alternation of the kinesin-1 and Eg5 heads, and a monastrol-dependent dwell time for the Eg5 component is shown by the dotted line. This line was generated by calculating Vpredicted=2Vk Ve/(Vk+Ve) for each monastrol concentration, where Vk is the measured MT sliding velocity for kinesin-1 and Ve is that for Eg5. The dotted line is the fit of the above inhibition equation to these calculated points. The actual behaviour (squares) is different, emphasizing that a scheme with strict alternation does not apply. MT, microtubule.