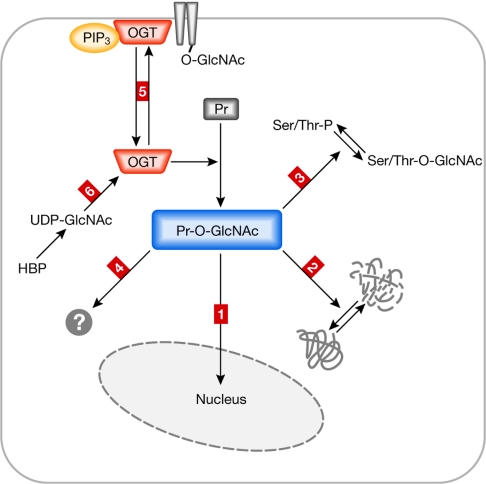
Figure 2.
Possible mechanisms controlling O-linked N-acetylglucosaminyl-transferase activity and the consequences of O-linked N-acetylglucosamine modifications. O-GlcNAc protein modifications mediated by OGT have different outcomes, some of which are summarized in this figure. OGT itself is under the control of different regulatory mechanisms. On O-GlcNAc modification, proteins (for example, transcription factors) translocate into the nucleus (outcome 1). O-GlcNAc modification can affect protein stability (outcome 2). O-GlcNAc modification occurs on serine/threonine (Ser/Thr) residues that are also targets of protein phosphorylation (outcome 3); this can result in competitive regulation of target proteins with different outcomes. O-GlcNAc modification might have additional (not yet well-characterized) effects on target proteins such as structural consequences, effects on catalytic activity and hetero-homodimer formation (outcome 4). OGT function can be regulated by cellular re-localization, such as recruitment to the plasma membrane (outcome 5). OGT activity depends on the concentration of its substrate UDP-GlcNAc, which is strongly coupled to the metabolic state of the cells (outcome 6). HBP, hexosamine biosynthetic pathway; O-GlcNAc, O-linked N-acetylglucosamine; OGT, O-linked N-acetylglucosaminyltransferase; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; Pr, protein; Pr-GlcNAc, O-GlcNAc-modified protein.