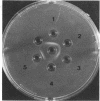
Abstract
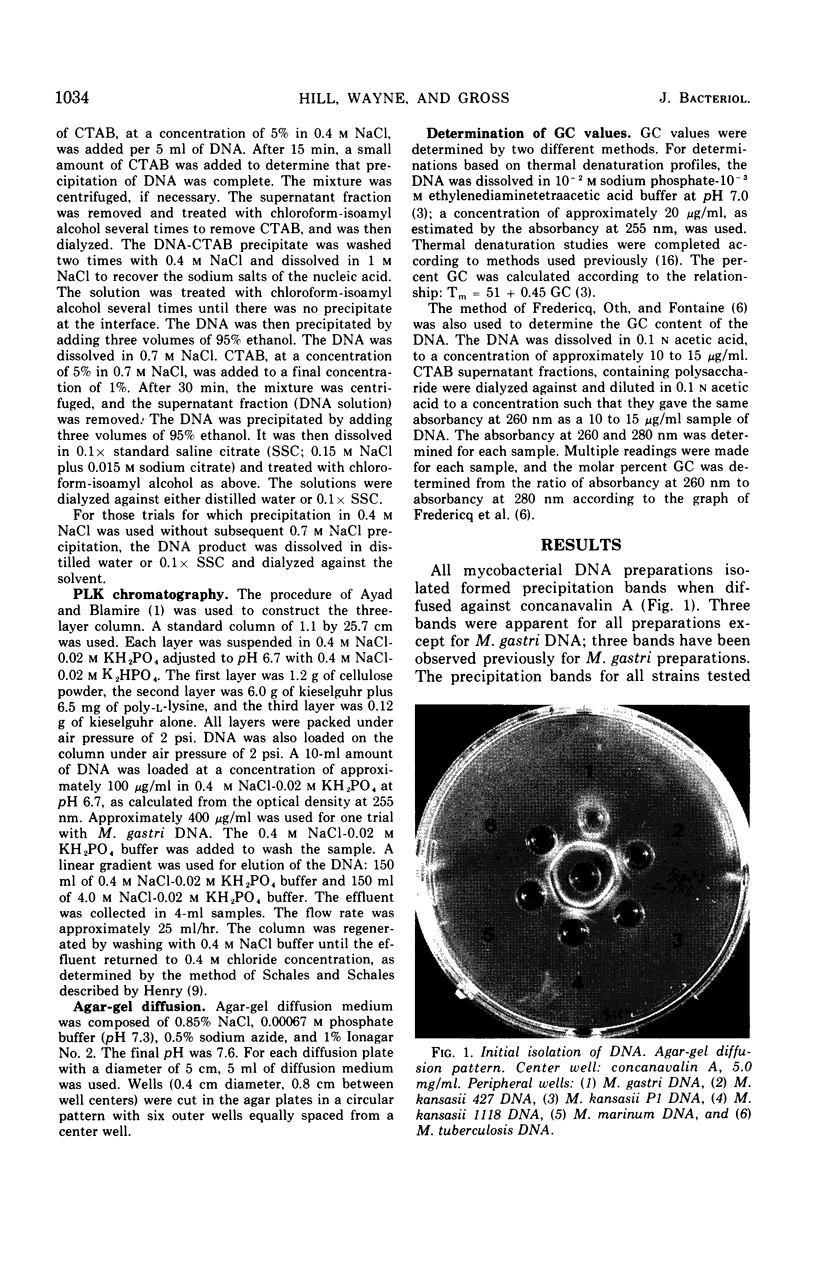
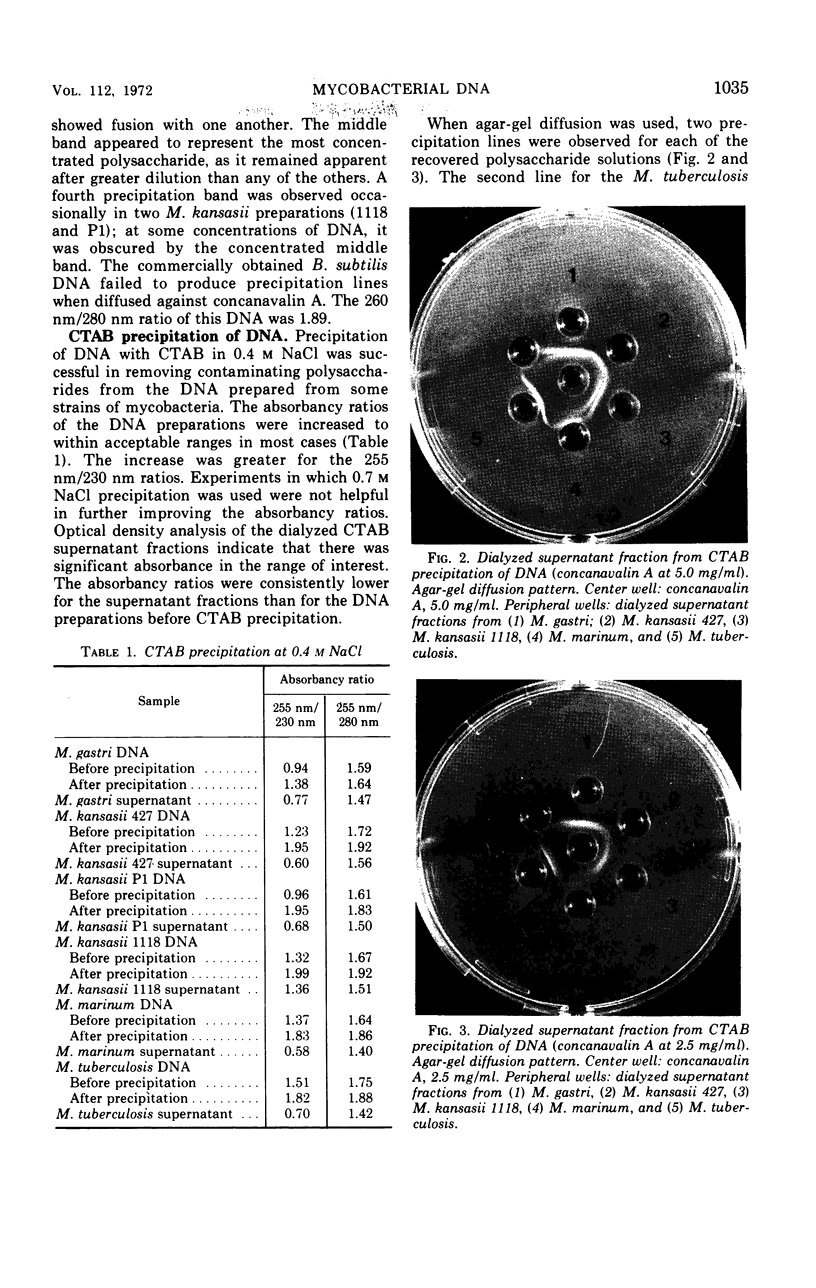
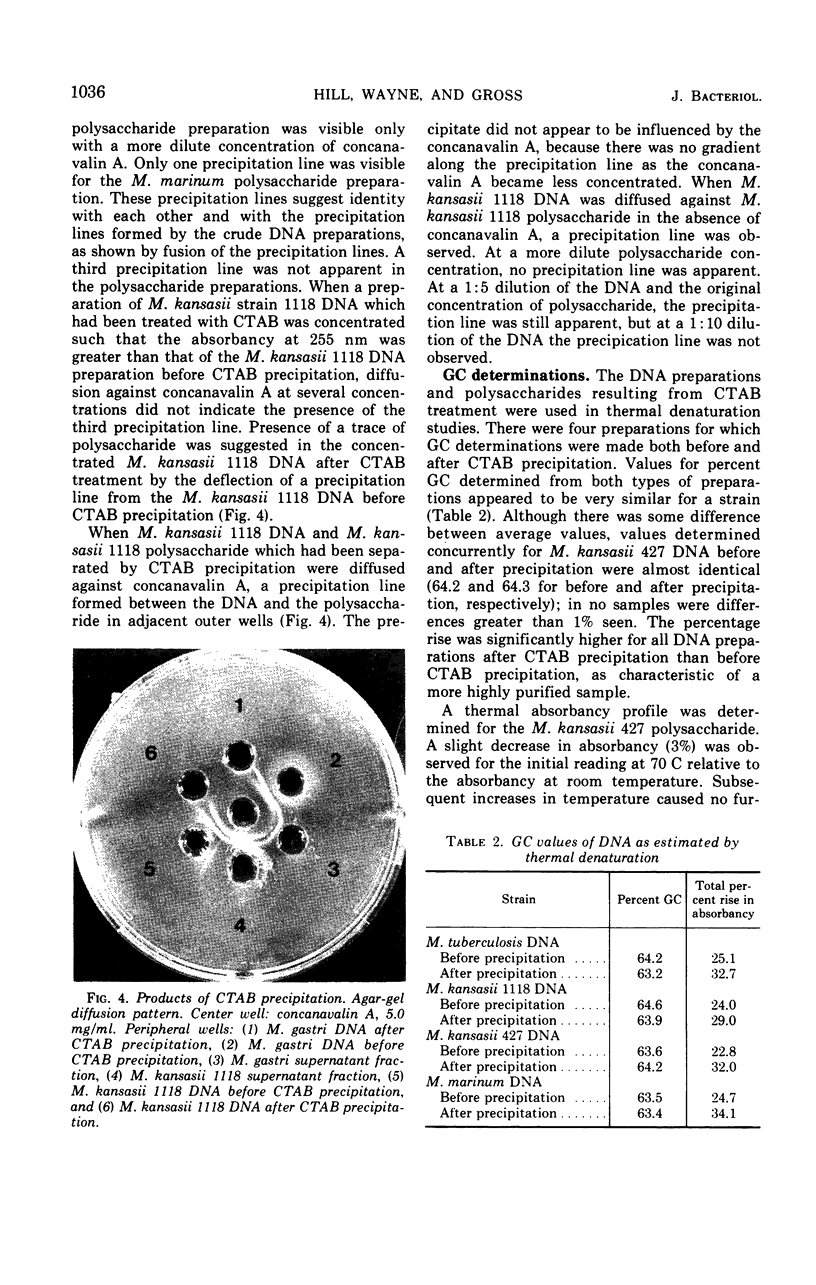
Impurities believed to be polysaccharides have been found in mycobacterial deoxyribonucleic acid (DNA) preparations. Agar-gel diffusion of the DNA preparations against concanavalin A indicated the presence of three polysaccharides and was used to follow the purification procedures. The polysaccharides appeared to be the same for all strains studied. Precipitation of DNA with cetyltrimethylammonium bromide was used to separate impurities from some DNA preparations. The presence of the contaminants was found to affect markedly the determination of the guanine plus cytosine content according to a method dependent on the ratio of absorbancies at 260 and 280 nm; the impurities did not affect the determination by the method of thermal denaturation. The presence of a DNA-polysaccharide complex is suggested.
Full text
PDF

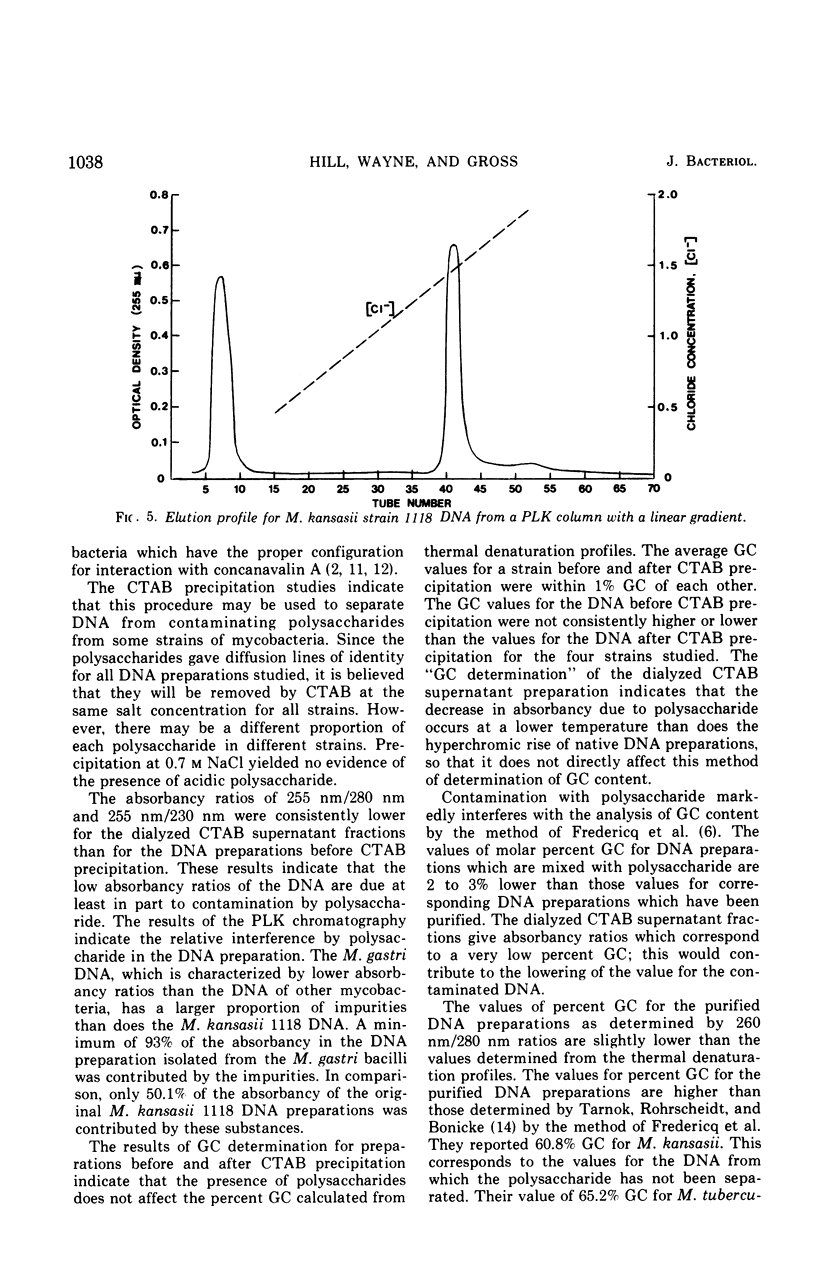

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayad S. R., Blamire J. Fractionation of Bacillus subtilis DNA by use of poly-L-lysine kieselguhr columns. Biochem Biophys Res Commun. 1968 Feb 15;30(3):207–212. doi: 10.1016/0006-291x(68)90436-1. [DOI] [PubMed] [Google Scholar]
- Azuma I., Ajisaka M., Yamamura Y. Polysaccharides of Mycobacterium bovis Ushi 10, Mycobacterium smegmatis, Mycobacterium phlei, and Atypical Mycobacterium P1. Infect Immun. 1970 Sep;2(3):347–349. doi: 10.1128/iai.2.3.347-349.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. M., Wisnieski J. J. The reaction of Concanavalin-A with mycobacterial culture filtrates. Am Rev Respir Dis. 1970 May;101(5):762–764. doi: 10.1164/arrd.1970.101.5.762. [DOI] [PubMed] [Google Scholar]
- Darby G. K., Jones A. S., Kennedy J. F., Walker R. T. Isolation and analysis of the nucleic acids and polysaccharides from Clostridium welchii. J Bacteriol. 1970 Jul;103(1):159–165. doi: 10.1128/jb.103.1.159-165.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ E., OTH A., FONTAINE F. The ultraviolet spectrum of deoxyribonucleic acids and their constituents. J Mol Biol. 1961 Feb;3:11–17. doi: 10.1016/s0022-2836(61)80003-x. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Misaki A. Interaction of concanavalin A with an arabinogalactan from the cell wall of Mycobacterium bovis. J Bacteriol. 1970 Aug;103(2):422–425. doi: 10.1128/jb.103.2.422-425.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleiner C. W. Fractionation of DNA on columns of poly-L-lysine supported on kieselguhr. Can J Biochem. 1969 Dec;47(12):1199–1202. doi: 10.1139/o69-193. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. IX. Application of the quantitative hapten inhibition technique to polysaccharide-concanavalin A interaction. Some comments on the forces involved n concanavalin A-polysaccharide interaction. J Immunol. 1967 Jul;99(1):158–163. [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Base composition of deoxyribonucleic acid isolated from mycobacteria. J Bacteriol. 1968 Dec;96(6):1915–1919. doi: 10.1128/jb.96.6.1915-1919.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Isolation of deoxyribonucleic acid from mycobacteria. J Bacteriol. 1968 Apr;95(4):1481–1482. doi: 10.1128/jb.95.4.1481-1482.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]