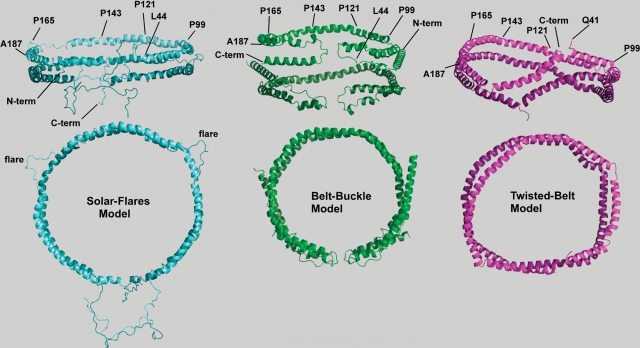
Fig. 1.
Three-dimensional models for two apoA-I molecules bound to ∼96 Å diameter POPC rHDL. Two views of lipidated apoA-I on recombinant HDL with the upper views illustrating an “on edge” view of the parallel strands of apoA-I for each model, whereas the second or lower view represents the top-down view. Phospholipid molecules have been left out of the figures for better visualization of protein conformation. Diameters, measured from the center of the helical strands, are given for each of the three-dimensional figures. Left: “Solar-flares” model showing the conformation for two apoA-I molecules bound to ∼104 Å diameter rHDL containing one hundred molecules of POPC to one full-length apoA-I molecule (75). The “solar-flares” name comes from the regions of apoA-I that are more solvent accessible. They are shown as flaring out into solution and correspond to amino acids 159–178. This model shows the N-terminal end extending into solvent away from the lipid, and the hydrophobic C-terminal helix 10 interacts with the 1–43 amino acid region also not associated with lipid (75). Coordinates were downloaded from the Protein Model Data Base, code PM0074956. Middle: “Belt-buckle” model showing the conformation for two apoA-I molecules bound to a 96 Å diameter rHDL containing seventy-five POPC molecules to one full-length apoA-I molecule (74). The N- and C-terminal ends are also closely associated, but in this model, fold back onto the central region of the apoA-I belt. In this way, the globular N-terminal domain of each apoA-I molecule folds or loops back onto itself, maximizing interaction with the C-terminal highly hydrophobic domain of apoA-I, functioning to adjust the “belt” in order to accommodate different amounts of phospholipids and cholesterol. Right: “Twisted-belt” model derived from molecular dynamic simulations of two apoA-I molecules bound to a 100 Å by 83 Å ellipsoidal rHDL containing eighty molecules of POPC to one truncated Δ40 apoA-I molecule (92). There is considerable agreement among these three three-dimensional models of lipid-bound apoA-I on rHDL. All models suggest that the central region of lipid-bound apoA-I assumes an antiparallel double belt with amphipathic helix 5 on each apoA-I strand situated adjacent to the same region of the second molecule of apoA-I. Differences between the models appear to involve the interaction of the N- and C-terminal regions of the two monomers.