Abstract
Proline and glycine residues are well represented among functionally important residues in hydrophobic domains of membrane transport proteins and several critical roles have been suggested for them. Here, the effects of mutational changes in membrane-embedded proline and glycine residues of Tet(L) were examined, with a focus on the conserved GP155,156 dipeptide of Motif C, a putative “antiporter motif”. Mutation of Gly155 to cysteine resulted in a mutant Tet(L) that bound its tetracycline-divalent metal (Tc-Me2+) substrate but did not catalyze efflux or exchange of Tc-Me2+ or catalyze uptake or exchange of Rb+ which was used to monitor the coupling ion. These results support suggestions that this region is involved in the conformational changes required for translocation. Mutations in Pro156 resulted in reduction (P156G) or loss (P156A or C) of Tc-Me2+ efflux capacity. All three Pro156 mutants exhibited a K+ leak (monitored by 86Rb+ fluxes) that was not observed in wild type Tet(L). A similar leak was observed in a mutant in a membrane-embedded proline residue elsewhere in the Tet(L) protein (P175C) as well as in a P156C mutant of related antiporter Tet(K). These findings are consistent with roles proposed for membrane-embedded prolines in tight helix packing. Patterns of Tc-resistance conferred by additional Tet(L) mutants indicate important roles for another GP dipeptide in transmembrane segment (TMS) X as well as for membrane-embedded glycine residues in TMS XIII.
Almost all transport proteins have membrane-embedded proline residues in some of the multiple α-helices that are a general feature of transporters (1). These residues are preferentially paired with glycine residues that are also found in abundance in TMS1 of transporters (2, 3). Membrane-embedded proline and glycine residues of transporters are hypothesized to promote helix kinks and swivels that play crucial roles in the conformational flexibility required for transport mechanisms (2-6). They are further hypothesized to have roles in helix packing and association (7-9). These hypotheses have been stunningly supported for the largest family of membrane transport proteins, the MFS, a family of structurally related transporters of prokaryotes and eukaryotes that includes uniporters, antiporters and symporters. The MFS comprises about a quarter of all transport proteins (10, 11). The first three high resolution structures of MFS members, the oxalate/formate antiporter OxlT (from Oxalobacter formigenes) (12), the lactose/proton symporter LacY (from Escherichia coli) (13) and the inorganic phosphate/glycerol-3-phosphate antiporter GlpT (from E. coli) (14), show clearly that many of the 12-TMS of these transporters are highly curved, with several of them having evident kinks. The positions of kinking in LacY are associated with 6 prolines, 3 glycines and 2 alanines (15).
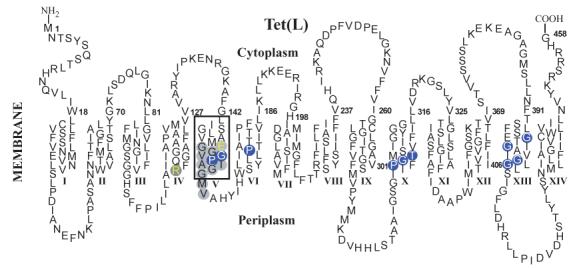
Antiporters of the MFS have a particular proline- and glycine-rich motif in TMS V that is designated Motif C and is one of several motifs that have been identified in 12-TMS and/or 14-TMS members of the superfamily (16-19) (Figure 1). Motif C has been a focus of interest in structure-function studies of both 12-TMS and 14-TMS tetracycline (Tc) antiporters, which are Tet proteins that catalyze efflux of a Tc-Me2+ complex from the cytoplasm in exchange for external H+ (20-22). Active Tc-Me2+ efflux by Tet proteins depends upon the Δp established across the cytoplasmic membrane by ATP- or respiration-dependent proton pumping. Complete cysteine-scanning mutagenesis of the 12-TMS TetA(B) antiporter from Gram-negative transposon Tn10 demonstrates that proline and glycine residues constitute a large proportion of the essential residues. The mutagenesis analysis further suggests that the conserved GP dipeptide of Motif C is located at a “permeability barrier” where conformational switching is likely to occur during the transport cycle (23-26). The importance of the GP peptide and several additional glycine residues of Motif C for Tc transport has also been shown in the Tet(K) protein by assays of mutants with diverse substitutions at these positions (27). Tet(K) and closely related Tet(L) form a class of 14-TMS Tet proteins in the MFS (28, 29). Both genes are found in plasmids of Gram-positive bacterial species, e.g. Staphylococcus aureus, with Tet(L) also being encoded in the chromosome of some Bacillus subtilis strains (19, 30, 31). Tet(L) and Tet(K) share sequence similarity to the 12-TMS Tet proteins in the N-terminal domain comprising the first six TMS, but structure-function differences in the two groups are evident even in this domain, including the presence of an essential acidic residue in the Motif C of Tet(L) and Tet(K) that is not conserved in the Motif C of 12-TMS Tet proteins (17).
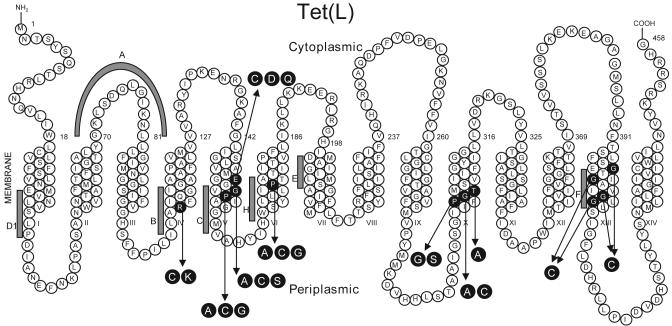
Figure 1.
A topological model of Tet(L) highlighting the residues functionally probed in this study. The topology is based on the experimental work of others on Tet(K) (28, 29). The gray bars indicate the positions of Motifs described by Paulsen et al. (17) that are conserved either in both 12-TMS and 14-TMS Drug/H+ families (Motifs A,B,C) or conserved only in 14-TMS (Motifs D1, H, E, F).
The 12- and 14-TMS Tet proteins are hypothesized to have evolved independently from each other on the basis of genomic considerations (32-34). They also have apparent functional differences. Only Tc-Me2+/H+ antiport has been demonstrated for 12-TMS Tet proteins and the antiport is electroneutral, with no flux of charge occurring during an antiport turnover (35). By contrast, Tet(L) and Tet(K) proteins catalyze Na+(K+)/H+ antiport, that supports alkaline pH homeostasis and Na+-resistance, in addition to the Tc-Me2+ /H+ antiport that supports antibiotic-resistance. All the antiport reactions catalyzed by Tet(L) and Tet(K) are electrogenic i.e. more than one H+ is translocated inward per Na+, K+ or mono-cationic Tc(−1)-Me2+ complex translocated outward (36-39) in contrast to the finding with the 12-TMS Tn10 Tet protein (35). Both Tet(L) and Tet(K) can also substitute K+ for part of the H+ coupling ion complement, especially at high pH, thus catalyzing both net K+ and H+ uptake under particular conditions of pH and K+ status (40-42). This use of K+ makes it possible to track the movement of the coupling ion (via inward fluxes of 86Rb+) in a direct manner that is not possible when only H+ is used as coupling ion (40).
In the present study, we sought to apply the experimental advantage of the inward 86Rb+ flux assay to complement Tc-Me2+ efflux assays in probing the role of the Motif C GP dipeptide in 14-TMS Tet proteins. In addition, we examined the effects on Tc-Me2+ efflux of two new mutations at another important residue of Tet(L) Motif C, E152D and E152C, and one new mutation at a site in the periplasmic half of TMS IV that is thought to interact with Motif C, R110D (24, 25). E152Q and R110C mutations were earlier shown to result in complete loss of tetracycline-resistance (41). Finally, we tested the effect on tetracycline-resistance of Tet(L) mutations in membrane-embedded glycines and prolines of two additional regions that are in the C-terminal domain of Tet(L): (i) a distinct GP dipeptide in TMS X that is in a functionally important region of a B. subtilis multi-drug resistance protein (43); and (ii) a glycine-rich region of TMS XIII, another helix that contains an essential acidic residue in Tet(K) and Tet(L) (41, 44). The residues studied are highlighted in Figure 1.
MATERIALS AND METHODS
Bacterial strains and growth conditions
The following E. coli strains were used: E. coli DH5α [F−ϕ80ΔlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) supE44 λ− thi-1 gyrA relA1] (GIBCO-BRL)(45) was used for cell and vesicles assays of Tc resistance and transport, respectively; E. coli JM109 and CJ236 (New England Biochemical) were used for steps of the site-directed mutagenesis as described previously (40); and E. coli TK2420 (trkD1 Δ(trkA) Δ(kdpABC)5 kup), a K+-uptake-deficient triple mutant (46), was used for assays of Rb+ uptake by right-side-out (RSO) vesicles. E. coli strains were routinely maintained and grown in LBK (47).
Construction and cloning of tet(L) and tet(K) genes with site-directed mutations
Mutations were introduced into the chromosomal tet(L) gene from B. subtilis and the S. aureus plasmid pT181 tet(K) gene which had earlier been cloned into the bacteriophage vector M13mp19 (40); phage DNA was used as template for site-directed mutagenesis by the method of Kunkel et al. (48). The mutants used in the study and the primers employed in their construction are shown in Table 1. Each construct was cloned into the shuttle vector pBK15 (obtained from K. Zen), which contains EmrCmr markers, a pBD42-pBR322 joint replicon and a multiple cloning site. The tet genes were cloned under control of the ermC promoter; experiments were conducted without added Em using the modest level of basal expression. The sequence of each construct was verified by sequencing of the whole tet ORF in the institutional DNA Core Facility.
Table 1.
New mutational changes made in Tet(L) and Tet(K)a
| Protein | Mutant | Primer Sequence 5'-3' |
|---|---|---|
| Tet(L) | E152D | CTGGTAGCAATGGGAGATGGTGTTGGGCCAGCT |
| G155A | ATGGGAGAAGGTGTTGGCCCAGCTATTGGCGGA | |
| G155C | ATGGGAGAAGGTGTTTGCCCAGCTATTGGCGGA | |
| G302A | GGCATTATTTTCCCCGCAACAATGAGTGTCATCA | |
| G302C | GGCATTATTTTCCCCTGTACAATGAGTGTCATCA | |
| G398C | AGCTTTTTATCAGAGTGTACGGGTATTGCGA | |
| G400C | TTATCAGAGGGAACGTGTATTGCGATTGTAGGC | |
| G405C | GGTATTGCGATTGTATGCGGTTTATTATCTATCGG | |
| G406C | ATTGCGATTGTAGGCTGTTTATTATCTATCGGCTTC | |
| P121C | GCAGCCGCATTCTGCGCTCTTGTGATGGTT | |
| P156A | GGAGAAGGTGTTGGGGCAGCTATTGGCGGAATG | |
| P156C | GGAGAAGGTGTTGGGTGTGCTATTGGCGGAATG | |
| P156G | GGAGAAGGTGTTGGGCCAGCTATTGGCGGAATG | |
| P175A | TATTTGCTGCTTATTGCAACTGCAACAATTATC | |
| P175G | TATTTGCTGCTTATTGGAACTGCAACAATTATC | |
| P183C | TATTTGCTGCTTATTTGCACTGCAACAATTATC | |
| P301S | AGCGGCATTATTTCCTCCGGAACAATGAGTGTC | |
| R110K | ATTCTCATTCTAGCCAAATTTATTCAAGGAATTGG | |
| T303A | CATTATTTTCCCCGGAGCAATGAGTGTCATCATCTT | |
| Tet(K) | G155C | CTTTAGGTGAAGGGTTATGTCCTTCAATAGGGGGA |
| G155S | CTTTAGGTGAAGGGTTAAGTCCTTCAATAGGGGGA | |
| P156C | GGTGAAGGGTTAGGTTGTTCAATAGGGGGAATA |
The bold letters in the primer sequence indicate the mutation introduced.
Assays of Tc-resistance of E. coli transformants
For assays of Tc-resistance, 10 μl of stationary phase cultures of E. coli DH5α transformants (with control or recombinant pBK15 plasmids) were inoculated into 2 ml of LBK containing various Tc concentrations in the range of 0-32 μg/ml. The A600 after 15 hours was plotted for determination of MIC (49). All assays were carried out in duplicate in at least two independent experiments. MIC values in this study are taken to represent Tc-resistance that is: negligible at ≤ 4 μg/ml; significantly defective at 5-12 μg/ml; and substantial at > 15 μg/ml. MIC values for Tc assayed in vivo do not correlate precisely with transport levels assessed in vitro (49, 50), but the semi-quantitative correlation between the lowest and highest group of values is strong.
Preparation of membrane vesicles
Everted vesicles were prepared from E. coli transformants in 10 mM BTP at pH 7.5 using a French Pressure cell method of Rosen (51) as described previously (40). RSO membrane vesicles were prepared by the lysozyme-method of Kaback (52) and were pre-loaded with either 100 μM choline chloride or KCl (42). Protein assays were conducted by the method of Lowry et al. (53) using egg white lysozyme as the standard.
Western analyses of membranes from E. coli transformants
After everted membrane samples (50 μg protein) were fractionated on sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membranes, Western analyses were carried out using antibodies raised to synthetic peptides corresponding to the N-terminus of Tet(L) or Tet(K), as described earlier (49). The signals were quantified by using ImageQuant software (Molecular Dynamics).
Transport assays in membrane vesicles of E. coli.
(i) Assessment of Tc-Co2+ efflux capacity by assays of energy (D-lactate)-dependent uptake of Tc-Co2+ into everted (inside-out) vesicles
Tc uptake was assayed at 30 C. in reaction mixtures containing 50 μg of vesicle protein in 10 mM BTP buffer, pH 7.5, 25 μM [3H]Tc and 100 μM CoCl2. Energized reactions also contained 5 mM Tris-D-lactate (an electron donor to the respiratory chain via membrane associated D-lactate dehydrogenase); this was included in the reaction mix so that the vesicles were energized when the substrate was added. The reaction was initiated by addition of [3H]Tc-CoCl2 from a 50x pre-mixed stock (1.25 mM Tc, 5 mM CoCl2) that allowed formation of the substrate complex. At intervals after initiation of the reaction, samples were filtered GSWP 02500 filters (Millipore), washed with cold reaction buffer, dried and assayed by liquid scintillation spectrometry. Parallel de-energized control reactions were conducted for each type of transformant membrane preparation in the absence of Tris-D-lactate and with addition of the uncoupler CCCP to a final concentration of 10 μM. Substrate binding under these de-energized conditions was subtracted from values obtained in the presence of D-lactate and absence of CCCP. Vector controls were also conducted routinely.
(ii) Assays of [3H]Tc-Co2+:Tc-Co2+ and K+-86Rb+: K+-Rb+ exchange in RSO vesicles
RSO vesicles were prepared in 10 mM BTP buffer, pH 7.5. For Tc-Co2+:Tc-Co2+ exchange they were passively loaded with 50 μM CoCl2 and 25 μM Tc, or with 50 μM cholineCl. Samples were diluted 1:10 in buffer containing 50 μM CoCl2 and 25 μM [3H]Tc. At various times, 50 μl samples, each containing 50 μg of vesicle protein, were filtered onto HAWP (0.45 μm) filters (Millipore) and radioactivity was monitored as described above. For K+-86Rb+: K+-Rb+ exchange, the vesicles were loaded with either 100 μM KCl or 100 μM cholineCl. They were diluted and assayed as for the other exchange except that the dilution buffer contained 100 μM K+-86Rb+. The binding control assays were conducted identically to the two exchange reactions except that the reaction mixtures contained 2% toluene to permeabilize the vesicles. Correction was made for the choline controls at each point of the assay and the data for the vector control are shown.
(iii) Assays of energy-dependent uptake of 86Rb+ into RSO vesicles and its dependence on the presence of a Tet(L) or Tet(K) efflux substrate inside the vesicle
RSO vesicles from E. coli TK2420 transformants were prepared in 10 mM BTP buffer, pH 7.5, and were passively loaded with either 100 μM cholineCl (non-efflux substrate for Tet(L) or Tet(K)) or 100 μM KCl (an efflux substrate). Assays of Tet(L)- and Tet(K)- 86Rb+-K+ uptake were initiated by dilution of 25 μl of the vesicle suspension into 500 μl of100 mM Tris-HCl (pH 7.5), containing 10 mM Tris-D-lactate and 100 μM 86Rb+-K+. The final membrane concentration was 500 μg membrane protein/reaction mixture. Binding controls were carried out without added Tris-D-lactate and with the addition of 5% butanol; butanol was empirically found to yield more consistent control values in this particular assay than the 2% toluene used for the exchange assays. At intervals, samples were filtered and processed as described under (i) except that HAWP (0.45 μm) filters (Millipore) were used.
RESULTS
Mutants of Arg110 in TMS IV and of Glu152 and Gly155 in the Motif C GP dipeptide
Earlier indications of the importance of Arg110 and Glu152 (41) were followed up with new mutants before the effort focused on the Motif C GP dipeptide and other Gly and Pro residues in TMS domains. A new R110K mutation of Tet(L), a more conservative change than the original R110C mutation, allowed greater membrane assembly than the original mutation, but no significant Tc-resistance was conferred (Table 2). Transport assays showed that the initial rate of [3H]Tc-Co2+ uptake by Tet(L) R110K vesicles was indistinguishable from that of wild type Tet(L), but was followed by cessation of uptake after 30 seconds, which was not observed in the wild type (data not shown). This uptake deficit probably accounts for the negligible Tc-resistance conferred by this mutant and might result from a coupling defect. By contrast, a charge-preserving mutation of Glu152 in Motif C, E152D, conferred significant Tc-resistance that was still defective relative to wild-type but also exhibited less membrane assembly than wild type; E152C and E152Q mutants of Tet(L) did not confer Tc-resistance. Transport assays showed that the [3H]Tc-Co2+ uptake activity of Tet(L) E152D in everted membrane vesicles and 86Rb+ uptake activity in RSO vesicles were comparable to the wild type activity, whereas the Tet(L) E152C mutant exhibited no Tet(L)-dependent transport in either assay (data not shown). Presumably a negative change is required at this position.
Table 2.
Effects of the Tet(L) and Tet(K) mutations on protein assembly into the membrane and Tc-resistance in E. coli DH5αa
| Tet(L) or Tet(K) |
Location of mutation | Protein property | % Membrane Assemblyb |
MIC of Tcc (μg/ml) |
|---|---|---|---|---|
| - | - | None (vector control) |
- | 2 |
| Tet(L) | None | Wild-type | 100 | 30 |
| Motif B, TMS IV | R110Cd | 18 | 2 | |
| R110K | 82 | 4 | ||
| Motif C, TMS V | E152C | 30 | 2 | |
| E152D | 49 | 12 | ||
| E152Qd | 98 | 3 | ||
| G155A | 134 | 16 | ||
| G155C | 100 | 8 | ||
| G155S | 152 | 8 | ||
| P156A | 60 | 2 | ||
| P156C | 94 | 2 | ||
| P156G | 121 | 2 | ||
| Motif H, TMS VI | P175A | 89 | 8 | |
| P175Cd | 88 | 16 | ||
| P175G | 74 | 16 | ||
| TMS X | P301G | NDe | >32 | |
| P301S | 86 | 2 | ||
| G302A | 76 | 8 | ||
| G302C | 62 | 2 | ||
| T303A | 66 | 8 | ||
| TMS XIII | G398C | 80 | 16 | |
| G400C | 73 | 8 | ||
| G405C | 60 | 2 | ||
| G406C | 65 | 8 | ||
| Tet(K) | Motif C, TMS V | Wild-type | 100 | 12 |
| G155C | 105 | 4 | ||
| G155S | 88 | 8 | ||
| P156C | 98 | 2 | ||
All values are the average of duplicate determinations in at least two independent experiments, with standard deviations ≤15% of the mean.
Percentage of the wild-type values (set at 100) as evaluated by Western analyses.
MIC is minimal Tc concentration at which there was no growth of the E. coli DH5α transformant after a 15-h incubation in rich medium (41).
These values have been reported previously , and are shown here for comparison.
Not determined.
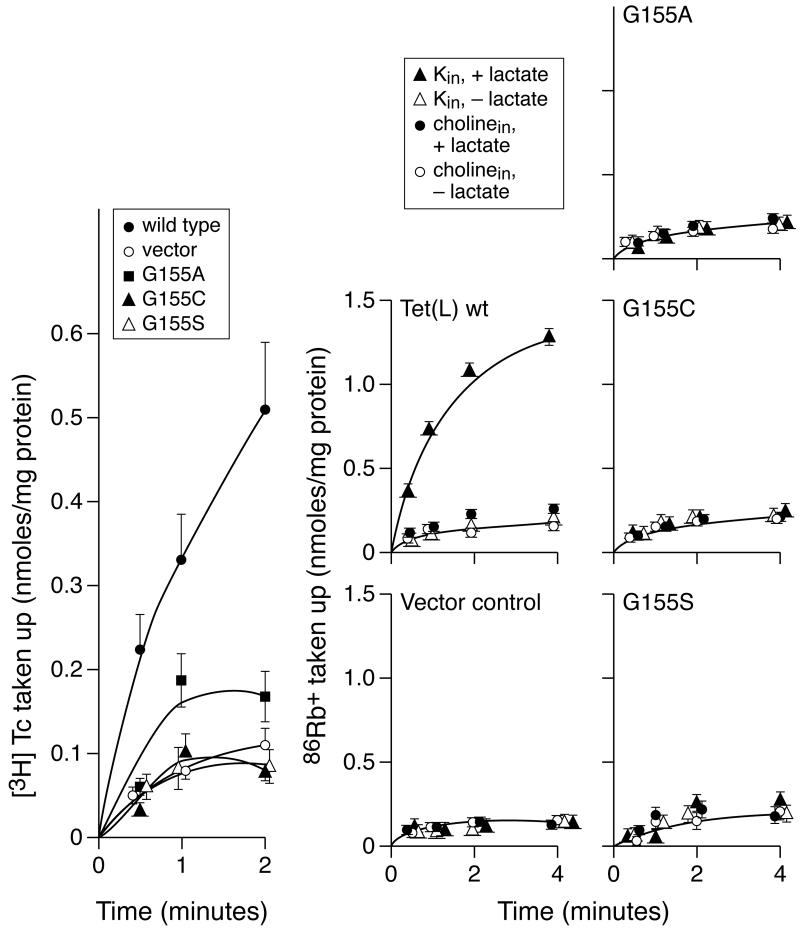
Three Tet(L) proteins with mutations of Gly 155 of the GP155,156 dipeptide (G155A, C, or S), all assembled at wild type levels or greater in the membranes of E. coli when expressed from a multi-copy plasmid (Table 2). Tet(L) G155A conferred substantial Tc-resistance while the G155C and G155S mutants were more deficient by this assessment (Table 2). Assays of [3H]Tc-Co2+ uptake by energized everted vesicles, the surrogate assay of Tc efflux by right-side-out cell or vesicle systems, confirmed that the Tet(L) G155A retained significant, low activity whereas the G155C and G155S mutants did not (Figure 2). None of the mutants exhibited any activity in assays of 86Rb+ uptake in RSO membrane vesicles; in these assays, K+-86Rb+ is used as part of the extravesicular coupling ion complement that exchanges for an intravesicular substrate of the transporter (Figure 2). The values shown in Figure 2 were corrected for binding controls conducted for each vesicle preparation. A consistent difference in the magnitude of [3H]Tc-Co2+ binding was observed between the two inactive Gly155 mutants, G155C and G155S, and the wild type Tet(L); there was no significant difference between the binding control of G155A and wild type. The binding assessed using uncoupler (CCCP)-treated vesicles averaged 0.05 ± 0.04 nmoles Tc/mg membrane vesicle protein in the wild type vesicles whereas the G155C vesicles averaged 0.25 ± 0.04 nmoles Tc/mg membrane protein; the same binding difference was observed when butanol treatment was used in addition to CCCP-treatment. Two experiments were conducted to assess the nature of this binding. First, the binding of [3H]Tc alone, under the same conditions except for the absence of Co2+, was assayed and found to be identical and in the low 0.05 nmoles/mg membrane protein in both wild type and G155 mutant membranes. Second, experiments were conducted in which the vesicles were incubated in a standard reaction mix containing [3H]Tc-Co2+ and then washed with buffer containing 100 μM non-radioactive Tc-Co2+ using a protocol described by others (54); no reduction in the membrane-associated [3H]Tc was found. No such difference in binding, as compared to wild type, was observed with any other Tet(L) mutant in this study.
Figure 2.
Transport assays of Tet(L) Gly155 mutants in comparison with wild type Tet(L). Left panel: Uptake of [3H]Tc-Co2+ into everted vesicles from the indicated transformants of E. coli DH5α were carried out as described under Materials and Methods. The values shown, with standard deviations, are the averages of duplicate determinations on at least two independent vesicle preparations. The values shown were corrected for the binding control of de-energized (CCCP-treated) vesicles from each of the same strains. Right panels: Uptake of 86Rb+ was assayed in RSO vesicles of transformants expressing the same plasmids used in assays of [3H]Tc-Co2 uptake but the bacterial strain was E. coli TK2420. The results for each transformant are shown in separate panels because assays were conducted under several different conditions that distinguish an electrogenic leak from antiport (the conditions are listed in the box above the wild type panel).
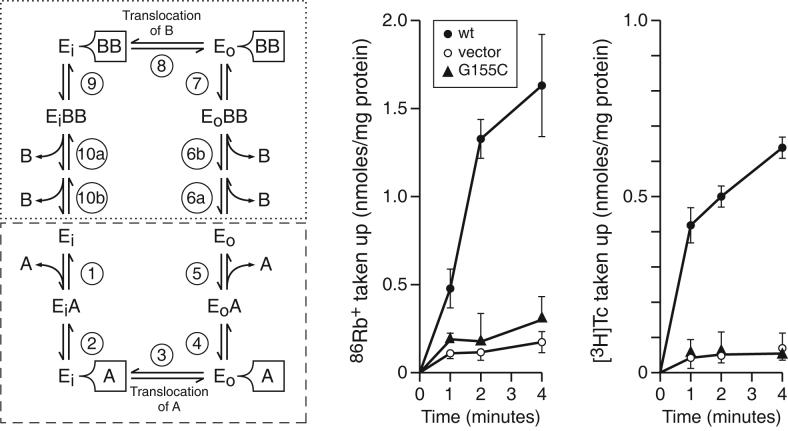
The profile of two Tet(L) Gly155 mutants suggested that these mutant Tet(L) forms were able to bind the cytoplasmic efflux substrate but were unable to carry out a subsequent step in the transport cycle. Assays of both 86Rb+:Rb+ exchange and [3H]Tc-Co2+: Tc-Co2+ exchange were conducted on wild type Tet(L) and the Tet(L) G155C mutant. These two exchange reactions, respectively, involve flux across the membrane of the radiolabeled coupling ion (into RSO vesicles) or substrate (into everted vesicles), dissociation of the coupling ion or substrate from Tet(L) and then transport of unlabelled coupling ion or substrate across in the return direction. Each exchange reaction therefore assays a segment of the complete reaction cycle that includes a conformational switch and that can support bi-directional flux of either the coupling ion or of the substrate across the membrane (in the absence of a complete substrate efflux cycle). As depicted in the model in Figure 3 (left), the [3H]Tc-Co2+: Tc-Co2+ exchange and 86Rb+:Rb+ exchanges respectively assay back and forth flux through steps 1-5 and 6-10. As shown in Figure 3 (middle and right), wild type Tet(L) exhibited activity in both exchange reactions but Tet(L) G155C exhibited neither exchange activity.
Figure. 3.
Assays of [3H]Tc-Co2+:Tc-Co2+ and K+-86Rb+: K+-Rb+ exchange in RSO vesicles expressing Tet(L) G155C. Left: A diagram of the reaction cycle for Tet(L): Ei and Eo respectively represent the inward and outward facing conformations of the transporter; A is the efflux substrate (e.g. Tc-Co2+) and B is a coupling ion which could be either H+ or Rb+-K+. The B:A ratio is greater than unity (42); the precise stoichiometry is not known and is shown here as 2B:A for diagrammatic purposes. The reaction cycle illustrates an alternating access or ping-pong model in which efflux substrate A binds to Ei in step 1 followed by hypothetical tight binding (step 2) that may be detected in the G155C mutant because of a defect in subsequent translocation (step 3) that usually occurs immediately after tight binding. This is followed by hypothesized release from tight binding (step 4) and final substrate release (step 5). The Tc:Co2+ exchange reaction assesses this set of reactions (steps 1-5), starting with unlabeled Tc:Co2+ inside and labeled Tc:Co2+ outside and proceeding forward and reverse. A comparable half reaction, going in both directions, with the top half cycle (reactions 6-10, dotted box) is assessed by the K+-86Rb+: K+-Rb+ exchange. Middle and Right: the experimental data for the two exchange reactions. The assays were carried out as described under Materials and Methods in comparison with wild type Tet(L) and control membranes. The data are the averages from two independent preparations and are shown with standard deviations.
Mutants of Pro156 in the Motif C GP dipeptide
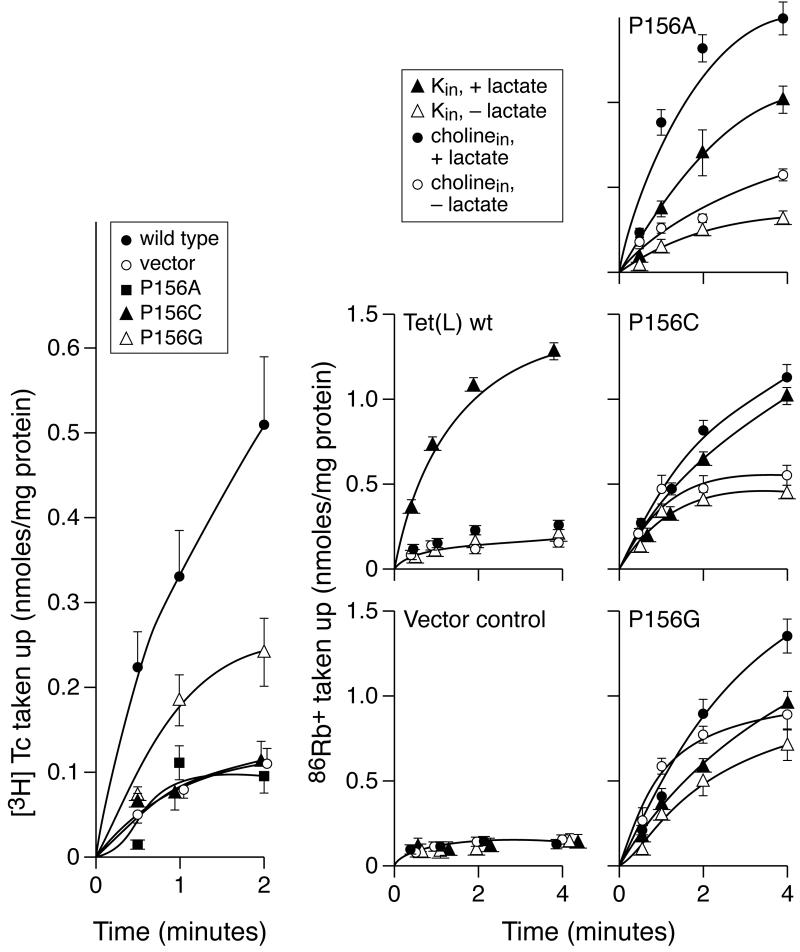
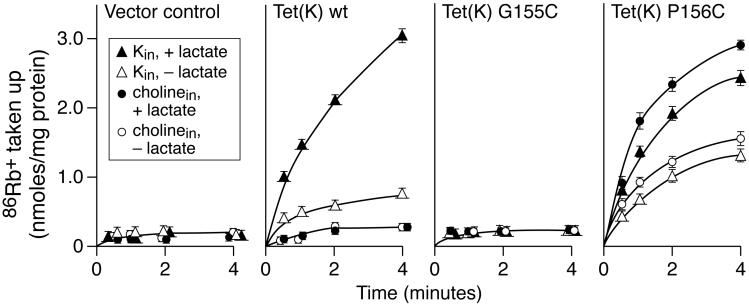
Mutation of Tet(L) Pro156 led to significantly reduced Tc-Co2+ efflux in the P156G mutant and to complete loss of efflux relative to the control for the P156A and P156C mutants (Figure 4). There was no indication of increased substrate binding in the assays. In the assays of 86Rb+ uptake, a remarkable the pattern was observed for all three Pro156 mutants. In this assay, 86Rb+ uptake into the RSO vesicles by wild type Tet(L) depends upon both the addition of the electron donor and on the presence of an efflux substrate for Tet(L) inside the vesicles; no Tet(L)-dependent 86Rb+ uptake was observed in the absence of added D-lactate (the electron donor) and or intravesicular K+ (the efflux substrate loaded inside the vesicles in this experiment) (Figure 4). Earlier work showed that the net Rb+-K+ uptake in this assay is a mode of the antiport that is electrogenic and it could be supported by the presence of either K+ or Tc-Co2+ inside the vesicles (40, 42). It was therefore notable that 86Rb+ uptake by the mutant Tet(L) P156A,G or C forms was as high or higher under conditions in which the electron donor was present but the intravesicular K+ was absent. This suggested that these mutant Tet(L) proteins promote leakage of K+ into the vesicles in response to the generation of the transmembrane potential, ΔΨ, while such a leak is not observed in wild type Tet(L) vesicles or with other Tet(L) mutants (e.g. see Figure 3) (40, 41, 49). The activity in the complete assay, with both electron donor and intravesicular K+, was lower than wild type in each Pro156 mutant (Figure 4).
Figure 4.
Transport assays of Tet(L) Pro156 mutants in comparison with wild type Tet(L). The assays were precisely as described in the legend to Figure 2.
Mutations in Gly155 and Pro156 of Tet(K)
In the study of Motif C mutants of Tet(K) by Ginn et al. (27), G155A, S and T mutants and P156A,V,G, and S mutants were all found to have residual but greatly reduced Tc-Co2+ transport activity. In assays of [3H]Tc-Co2+ uptake into everted vesicles under our conditions of expression and binding controls, the G155S mutant of Tet(K) exhibited 65% of wild type Tet(K) activity whereas the G155C and P156C mutants exhibited no activity (data not shown). Assays of 86Rb+ uptake by the P156C mutant of Tet(K) were carried out in comparison with wild type Tet(K) and the G155C Tet(K) mutant to assess whether mutations in Pro156 result in an apparent K+ leak as observed with Tet(L) mutants at this position. As shown in Figure 5, wild type Tet(K) exhibited a significantly greater rate of 86Rb+ uptake into RSO vesicles than Tet(L), as consistently observed before (40, 42), and this uptake was dependent upon energization and intravesicular K+. By contrast, Tet(K) G155C exhibited no 86Rb+ uptake under any condition. Tet(K) P156C exhibited a lower rate of 86Rb+ uptake than wild type in the complete assay mix and exhibited a significantly higher rate in the absence of intravesicular K+, consistent with a ΔΨ-dependent K+ leak.
Figure 5.
Assays of 86Rb+ uptake by RSO vesicles of E. coli TK2420 expressing wild type Tet(K) or Tet(K) mutants G155C or P156C. Assays were conducted as described for the right-hand panels of Figure 2.
Assessment of the K+-Rb+ leak vs antiport-dependent uptake in a Pro175 mutant
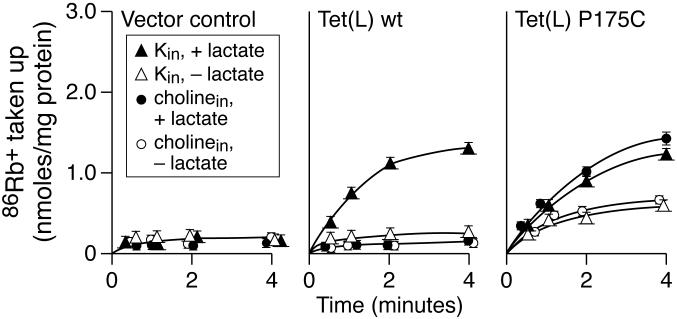
The apparent K+ leak conferred by mutations in Pro156 raised the question of whether such a leak would result from mutational change in a TMS proline residue outside of Motif C. Assays were conducted on Tet(L) P175C a mutant Tet(L) in a membrane-embedded proline residue of Motif H of proton-coupled drug efflux transporters (TMS VI, see Figure 1 for position) (17); this mutant Tet(L) had earlier been shown to be incorporated into the membrane and confer significant Tc-resistance (41). In assays of Tc-Co2+ uptake into everted vesicles, Tet(L) P175C vesicles had 43% of the wild type level of activity. Assays of 86Rb+ uptake by RSO vesicles expressing wild type Tet(L) vs Tet(L) P175C showed distinct differences in pattern that were similar to those observed for mutants in Pro156 (Figure 6). As always, 86Rb+ uptake by wild type Tet(L) depended upon both electron donor addition and the presence of intravesicular K+. By contrast, the Tet(L) P175C vesicles exhibited electron donor-dependent 86Rb+ uptake that was at least as rapid in the absence as in the presence of intravesicular K+ and the rate of transport was reduced relative to wild type Tet(L).
Figure 6.
Assays of 86Rb+ uptake by RSO vesicles of E. coli TK2420 expressing wild type Tet(L) or Tet(L) P175C. Assays were conducted as described for the right-hand panels of Figure 2.
Mutations in additional membrane-embedded Gly and Pro residues
The PG301,302 dipeptide of TMS X is modeled to be in a comparable position near the periplasmic end of the helix to the position of GP155,156 of TMS V in Tet(L) (Figure 1). Mutations Gly302 and neighboring Thr303 significantly reduced the Tc-resistance conferred by Tet(L), whereas two different mutations in Pro301 resulted in opposite effects, with P301G conferring greater Tc-resistance than wild type Tet(L) and P301S conferring no Tc-resistance (Table 2). Single cysteine replacements in a pair of similarly located glycines, Gly405,406, as well as of Gly400 in the Motif F of TMS XIII, greatly reduced Tc-resistance where as a mutant in Gly398 was less impaired.
DISCUSSION
The functional importance of Pro and Gly residues in several TMS domains of Tet(L) reinforces the finding of Tamura et al. (24) that among the thirteen essential residues that are in the TMS domains of the TetA(B) protein there are eight Gly and 2 Pro residues. The specific hypothesis that Motif C is important in antiporters of the Drug/H+ antiporter families (18) and that the conserved GlyPro155,156 dipeptide is involved in the “permeability barrier” (23) is supported by the effects of Gly155 mutations in the current study. The elevated binding of Tc by the de-energized transformant membranes of Tet(L) G155C and S, only when Co2+ was also present, indicates that substrate binding occurred in this mutant and that some subsequent step was arrested by the mutation. The absence of [3H]Tc-Co2+:Tc-Co2+ and 86Rb+:Rb+ exchange activity for the G155C mutant Tet(L) further suggests that the arrested step is the conformational switching in which movements in the region of the permeability barrier allow the substrate and the coupling ions to be translocated, in a ping-pong fashion (40), to the opposite sides from where they start. Apart from these structure-function inferences, a mutant that binds substrate but does not undergo major conformational movements could be useful for structural studies, as shown by the successful pursuit of a high resolution structure of LacY using a mutant, C154G, that binds substrate but is blocked in the conformational changes needed for translocation (13, 55).
As with Tet(L) G155C, de-energized control vesicles in transport assays of the TetA(B) G145C mutation (the equivalent position to Tet(L) G155 in the 12-TMS Tc-Me2+/H+ antiporter) as well as mutants in two other Motif C Gly residues of TetA(B), showed elevated binding compared to controls for other mutants (23). Tet(L) G155C or S retained a modest capacity to confer resistance in the absence of Tc-Co2+ efflux activity, just as Iwaki et al. (23) found for the similarly located G145C mutation of TetA(B) G145C. It is not known whether these findings represent an effect of these mutant Tet proteins on the E. coli host (e.g. increasing expression of E. coli transporters with Tc transport capacity or reducing Tc entry by modulating membrane properties) or whether these mutant Tet proteins may actually retain modest transport capacity under in vivo conditions of ion composition etc. that are not reproduced in vesicles. Both here and in the study of Tet(K) Motif C by Ginn et al. (27), significant transport activity was observed in a G155S mutant (and several other Gly155 mutants in the latter study) in contrast to the absence of activity in Tet(L) G155S. Probably, the closely related Tet(L) and Tet(K) have sufficient differences in conformation to account for small differences in mutational profiles. We note that although the Tet(L) G155A mutant retained some transport activity, no 86Rb+ uptake by that mutant was observed, suggesting that the mutational change may have lowered the preference for Rb+-K+ vs. H+ so that higher pH values would be required to observe participation of Rb+-K+ as coupling ion (Figure 2) (40).
The second major finding of the current study is that mutations in the conserved Pro156, whether they completely abolish Tc-Co2+ efflux or not, produce an electrogenic K+ leak, i.e. a Tet(L)-dependent, electron donor-dependent flux of 86Rb+ into RSO membrane vesicles in the absence of a pre-loaded efflux substrate (K+, Na+ or Tc-Co2+) inside the vesicles. This flux is thus not coupled to efflux of an intravesicular substrate, a coupling that is characteristic of antiport and is observed for such fluxes in wild type Tet(L) (40, 42). In assays of Tet(L) Pro156 RSO vesicles that were loaded with K+, enhancement of 86Rb+ uptake by the electron donor was observed with all three of the Pro156 mutants, and was particularly evident in the P156A mutant in the early time points (compare data shown with closed triangles with open triangles in right panels of Figure 4). The P156A and C mutants may retain some electrogenic monovalent cation/proton antiport activity although they were negative for Tc-Co2+ efflux. Several earlier mutants led to the hypothesis that although the efflux substrates share a binding site and translocation pathway, the most complicated substrate, the Tc-Co2+ complex, required more elements to position it properly than the simpler monovalent cation efflux substrates (40, 41, 49). Together, the current observations suggest that Pro156 is not essential for antiport but is very important in tight packing and “leak-proofing” in this critical region in both Tet(L) and Tet(K).
The packing function of Pro156 is likely to be a general feature of membrane-embedded proline residues since Tet(L) P175C exhibited a similar leak to the P156C mutants of Tet(L) and Tet(K). Prolines are not generally accommodated within membrane helices without deleterious effects and thus are likely to be functionally important when retained (56). It has been hypothesized that membrane-embedded proline residues are required for the initial formation of helix kinks but are often replaced during evolution by other residues, e.g. alanine, concomitant with adaptations that retain the kink and its function in the protein (57). Interestingly, Buurman et al. (58) suggested that multiple “aberrant” K+ fluxes function as electrogenic uniport activities in E. coli and may together comprise an observed low affinity K+ uptake capacity that has been termed TrkF. Perhaps some of these fluxes represent evolving transporters that are in the process of closing up small K+ leaks created as membrane-embedded proline residues were replaced. Part of this aggregate, low affinity K+ flux in E. coli is also likely to be comprised of antiporters that, like Tet(L), CzcD and others, are able to use both H+ and K+ as part of their coupling ion complement (42, 59, 60).
The data collected here on effects of mutations in residues of the Tet(L) PGT301-303 tripeptide of TMS X and Gly residues of TMS XIII indicate the importance of many of these residues in Tc-resistance. The results with Pro301 are particularly intriguing, since the Tet(L) P301G mutant and P301S mutants conferred, respectively, greater than wild type resistance and no resistance to Tc. These findings suggest that the PGT tripeptide of TMS X does not function identically to Motif C but that it is likely be involved in different conformational states of the transporter that require flexibility in the Pro301 position. Both this region and Motif F merit further study; Motif F is confined to 14-TMS Tet proteins (17) and has not yet been studied in detail in any Tet(L) or Tet(K). The significant defect in Tc-resistance that results from mutations of glycines in this motif, especially Gly405, suggest that this region too may be involved in the different conformational states of the transporter. Finally, the new mutations of Tet(L) R110K mutation and E152D mutation indicate that conservative mutations that preserve the original charge are tolerated better at the Glu152 position. Among Tet proteins, the Motif C Glu152 is only conserved in the Tet(L) and Tet(K) antiporters and even this may not be the rule. A new member of this group of Tet resistance proteins, Tet38, lacks Glu152 and also Pro156 (61); the catalytic properties of this protein will be of considerable interest.
For table of contents use only
Footnotes
The research was supported by NIH (RO1-GM052837). M. De Jesus was a scholar in the Post-Baccalaureate Research Education Program supported by National Institute of General Medical Sciences award R25-GM64118.
Abbreviations: BTP, bis-[tris(hydroxymethyl)methylamino]-propane; CCCP, cyanide-m-chlorophenylhydrazone; Δp, electrochemical proton gradient (inside negative and alkaline); Δψ, transmembrane electrical potential (inside negative); LBK, Luria Broth medium with KCl replacing NaCl; MFS, Major Facilitator Superfamily of membrane transporters; MIC, minimal inhibitory concentration; RSO, right side out membrane vesicles; Tc, tetracycline; Tc-Me2+, tetracycline-divalent metal complex; TMS, transmembrane segment.
REFERENCES
- 1.Brandl CJ, Deber CM. Hypothesis about the function of membrane-buried proline residues in transport proteins. Proc. Natl. Acad. Sci. USA. 1986;83:917–921. doi: 10.1073/pnas.83.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landolt-Marticorena C, Williams KA, Deber CM, Reithmeier RA. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J. Mol. Biol. 1993;229:602–608. doi: 10.1006/jmbi.1993.1066. [DOI] [PubMed] [Google Scholar]
- 3.Cordes FS, Bright JN, Sansom MS. Proline-induced distortions of transmembrane helices. J. Mol. Biol. 2002;323:951–960. doi: 10.1016/s0022-2836(02)01006-9. [DOI] [PubMed] [Google Scholar]
- 4.O'Neil KT, DeGrado WF. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990;250:646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- 5.Williams KA, Deber CM. Proline residues in transmembrane helices: structural or dynamic role? Biochemistry. 1991;30:8919–8923. doi: 10.1021/bi00101a001. [DOI] [PubMed] [Google Scholar]
- 6.West IC. Ligand conduction and the gated-pore mechanism of transmembrane transport. Biochim. Biophys. Acta. 1997;1331:213–234. doi: 10.1016/s0304-4157(97)00007-5. [DOI] [PubMed] [Google Scholar]
- 7.Javadpour MM, Eilers M, Groesbeek M, Smith SO. Helix packing in polytopic membrane proteins: role of glycine in transmembrane helix association. Biophys. J. 1999;77:1609–1618. doi: 10.1016/S0006-3495(99)77009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix-helix association. J. Mol. Biol. 2000;296:911–919. doi: 10.1006/jmbi.1999.3489. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Engelman DM, Gerstein M. Genomic analysis of membrane protein families: abundance and conserved motifs. Genome Biol. 2002;3:54. doi: 10.1186/gb-2002-3-10-research0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pao SS, Paulsen IT, Saier MH., Jr. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saier MH., Jr. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 2000;64:354–411. doi: 10.1128/mmbr.64.2.354-411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirai T, Heymann JA, Shi D, Sarker R, Maloney PC, Subramaniam S. Three-dimensional structure of a bacterial oxalate transporter. Nat. Struct. Biol. 2002;9:597–600. doi: 10.1038/nsb821. [DOI] [PubMed] [Google Scholar]
- 13.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 15.Abramson J, Iwata S, Kaback HR. Lactose permease as a paradigm for membrane transport proteins. Mol. Membr. Biol. 2004;21:227–236. doi: 10.1080/09687680410001716862. [DOI] [PubMed] [Google Scholar]
- 16.Varela MF, Sansom CE, Griffith JK. Mutational analysis and molecular modelling of an amino acid sequence motif conserved in antiporters but not symporters in a transporter superfamily. Mol. Membr. Biol. 1995;12:313–319. doi: 10.3109/09687689509072433. [DOI] [PubMed] [Google Scholar]
- 17.Paulsen IT, Brown MH, Skurray RA. Proton-dependent multidrug efflux systems. Microbiol. Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith JK, Baker ME, Rouch DA, Page MG, Skurray RA, Paulsen IT, Chater KF, Baldwin SA, Henderson PJ. Membrane transport proteins: implications of sequence comparisons. Curr. Opin. Cell Biol. 1992;4:684–695. doi: 10.1016/0955-0674(92)90090-y. [DOI] [PubMed] [Google Scholar]
- 19.Guillaume G, Ledent V, Moens W, Collard JM. Phylogeny of efflux-mediated tetracycline resistance genes and related proteins revisited. Microb. Drug Resist. 2004;10:11–26. doi: 10.1089/107662904323047754. [DOI] [PubMed] [Google Scholar]
- 20.McMurry L, Petrucci RE, Jr., Levy SB. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1980;77:3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMurry LM, Levy SB. Tetracycline resistance in Gram-positive bacteria. In: Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI, editors. Gram-positive pathogens. ASM Press; Washington D.C.: 2000. pp. 660–677. [Google Scholar]
- 22.Yamaguchi A, Udagawa T, Sawai T. Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J. Biol. Chem. 1990;265:4809–4813. [PubMed] [Google Scholar]
- 23.Iwaki S, Tamura N, Kimura-Someya T, Nada S, Yamaguchi A. Cysteine-scanning mutagenesis of transmembrane segments 4 and 5 of the Tn10-encoded metal-tetracycline/H+ antiporter reveals a permeability barrier in the middle of a transmembrane water-filled channel. J. Biol. Chem. 2000;275:22704–22712. doi: 10.1074/jbc.m910354199. [DOI] [PubMed] [Google Scholar]
- 24.Tamura N, Konishi S, Iwaki S, Kimura-Someya T, Nada S, Yamaguchi A. Complete cysteine-scanning mutagenesis and site-directed chemical modification of the Tn10-encoded metal-tetracycline/H+ antiporter. J. Biol. Chem. 2001;276:20330–20339. doi: 10.1074/jbc.M007993200. [DOI] [PubMed] [Google Scholar]
- 25.Tamura N, Konishi S, Yamaguchi A. Mechanisms of drug/H+ antiport: complete cysteine-scanning mutagenesis and the protein engineering approach. Curr. Opin. Chem. Biol. 2003;7:570–579. doi: 10.1016/j.cbpa.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Vardy E, Arkin IT, Gottschalk KE, Kaback HR, Schuldiner S. Structural conservation in the major facilitator superfamily as revealed by comparative modeling. Protein Sci. 2004;13:1832–1840. doi: 10.1110/ps.04657704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginn SL, Brown MH, Skurray RA. The TetA(K) tetracycline/H+ antiporter from Staphylococcus aureus: mutagenesis and functional analysis of motif C. J. Bacteriol. 2000;182:1492–1498. doi: 10.1128/jb.182.6.1492-1498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginn SL, Brown MH, Skurray RA. Membrane topology of the metal-tetracycline/H+ antiporter TetA(K) from Staphylococcus aureus. J. Bacteriol. 1997;179:3786–3789. doi: 10.1128/jb.179.11.3786-3789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirata T, Fujihira E, Kimura-Someya T, Yamaguchi A. Membrane topology of the staphylococcal tetracycline efflux protein Tet(K) determined by antibacterial resistance gene fusion. J. Biochem. (Tokyo) 1998;124:1206–1211. doi: 10.1093/oxfordjournals.jbchem.a022239. [DOI] [PubMed] [Google Scholar]
- 30.Amano H, Ives CL, Bott KF, Shishido K. A limited number of Bacillus subtilis strains carry a tetracycline-resistance determinant at a site close to the origin of replication. Biochim. Biophys. Acta. 1991;1088:251–258. doi: 10.1016/0167-4781(91)90061-p. [DOI] [PubMed] [Google Scholar]
- 31.Krulwich TA, Lewinson O, Padan E, Bibi E. Do physiological roles foster persistence of drug/multidrug-efflux pumps? 2005 doi: 10.1038/nrmicro1181. Manuscript submitted. [DOI] [PubMed] [Google Scholar]
- 32.Levy SB. Active efflux mechanisms for antimicrobial resistance. Antimicrob. Agents Chemother. 1992;36:695–703. doi: 10.1128/aac.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulsen IT, Skurray RA. Topology, structure and evolution of two families of proteins involved in antibiotic and antiseptic resistance in eukaryotes and prokaryotes--an analysis. Gene. 1993;124:1–11. doi: 10.1016/0378-1119(93)90755-r. [DOI] [PubMed] [Google Scholar]
- 34.Saier MH, Jr., Paulsen IT, Sliwinski MK, Pao SS, Skurray RA, Nikaido H. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 1998;12:265–274. doi: 10.1096/fasebj.12.3.265. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi A, Iwasaki-Ohba Y, Ono N, Kaneko-Ohdera M, Sawai T. Stoichiometry of metal-tetracycline/H+ antiport mediated by transposon Tn10-encoded tetracycline resistance protein in Escherichia coli. FEBS Lett. 1991;282:415–418. doi: 10.1016/0014-5793(91)80527-a. [DOI] [PubMed] [Google Scholar]
- 36.Cheng J, Guffanti AA, Wang W, Krulwich TA, Bechhofer DH. Chromosomal tetA(L) gene of Bacillus subtilis: regulation of expression and physiology of a tetA(L) deletion strain. J. Bacteriol. 1996;178:2853–2860. doi: 10.1128/jb.178.10.2853-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng J, Hicks DB, Krulwich TA. The purified Bacillus subtilis tetracycline efflux protein TetA(L) reconstitutes both tetracycline-cobalt/H+ and Na+(K+)/H+ exchange. Proc. Natl. Acad. Sci. USA. 1996;93:14446–14451. doi: 10.1073/pnas.93.25.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng J, Guffanti AA, Krulwich TA. The chromosomal tetracycline resistance locus of Bacillus subtilis encodes a Na+/H+ antiporter that is physiologically important at elevated pH. J. Biol. Chem. 1994;269:27365–27371. [PubMed] [Google Scholar]
- 39.Guffanti AA, Krulwich TA. Tetracycline/H+ antiport and Na+/H+ antiport catalyzed by the Bacillus subtilis TetA(L) transporter expressed in Escherichia coli. J. Bacteriol. 1995;177:4557–4561. doi: 10.1128/jb.177.15.4557-4561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin J, Guffanti AA, Bechhofer DH, Krulwich TA. Tet(L) and Tet(K) tetracycline-divalent metal/H+ antiporters: characterization of multiple catalytic modes and a mutagenesis approach to differences in their efflux substrate and coupling ion preferences. J. Bacteriol. 2002;184:4722–4732. doi: 10.1128/JB.184.17.4722-4732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin J, Krulwich TA. Site-directed mutagenesis studies of selected motif and charged residues and of cysteines of the multifunctional tetracycline efflux protein Tet(L) J. Bacteriol. 2002;184:1796–1800. doi: 10.1128/JB.184.6.1796-1800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guffanti AA, Cheng J, Krulwich TA. Electrogenic antiport activities of the Gram-positive Tet proteins include a Na+(K+)/K+ mode that mediates net K+ uptake. J. Biol. Chem. 1998;273:26447–26454. doi: 10.1074/jbc.273.41.26447. [DOI] [PubMed] [Google Scholar]
- 43.Klyachko KA, Neyfakh AA. Paradoxical enhancement of the activity of a bacterial multidrug transporter caused by substitutions of a conserved residue. J. Bacteriol. 1998;180:2817–2821. doi: 10.1128/jb.180.11.2817-2821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujihira E, Kimura T, Shiina Y, Yamaguchi A. Transmembrane glutamic acid residues play essential roles in the metal-tetracycline/H+ antiporter of Staphylococcus aureus. FEBS Lett. 1996;391:243–246. doi: 10.1016/0014-5793(96)00743-0. [DOI] [PubMed] [Google Scholar]
- 45.Grant SG, Jessee J, Bloom FR, Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA. 1990;87:4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epstein W, Buurman E, McLaggan D, Naprstek J. Multiple mechanisms, roles and controls of K+ transport in Escherichia coli. Biochem. Soc. Trans. 1993;21:1006–1010. doi: 10.1042/bst0211006. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg EB, Arbel T, Chen J, Karpel R, Mackie GA, Schuldiner S, Padan E. Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1987;84:2615–2619. doi: 10.1073/pnas.84.9.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunkel TA, Bebenek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 49.Jin J, Guffanti AA, Beck C, Krulwich TA. Twelve-transmembrane-segment (TMS) version (ΔTMS VII-VIII) of the 14-TMS Tet(L) antibiotic resistance protein retains monovalent cation transport modes but lacks tetracycline efflux capacity. J. Bacteriol. 2001;183:2667–2671. doi: 10.1128/JB.183.8.2667-2671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujihira E, Kimura T, Yamaguchi A. Roles of acidic residues in the hydrophilic loop regions of metal-tetracycline/H+ antiporter Tet(K) of Staphylococcus aureus. FEBS Lett. 1997;419:211–4. doi: 10.1016/s0014-5793(97)01457-9. [DOI] [PubMed] [Google Scholar]
- 51.Rosen BP. Ion extrusion systems in E. coli. Methods Enzymol. 1986;125:328–386. doi: 10.1016/s0076-6879(86)25028-4. [DOI] [PubMed] [Google Scholar]
- 52.Kaback HR. Bacterial membranes. Methods Enzymol. 1971;22:99–120. [Google Scholar]
- 53.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 54.Rudnick G, Steiner-Mordoch SS, Fishkes H, Stern-Bach Y, Schuldiner S. Energetics of reserpine binding and occlusion by the chromaffin granule biogenic amine transporter. Biochemistry. 1990;29:603–608. doi: 10.1021/bi00455a002. [DOI] [PubMed] [Google Scholar]
- 55.Smirnova IN, Kaback HR. A mutation in the lactose permease of Escherichia coli that decreases conformational flexibility and increases protein stability. Biochemistry. 2003;42:3025–3031. doi: 10.1021/bi027329c. [DOI] [PubMed] [Google Scholar]
- 56.Yohannan S, Yang D, Faham S, Boulting G, Whitelegge J, Bowie JU. Proline substitutions are not easily accommodated in a membrane protein. J. Mol. Biol. 2004;341:1–6. doi: 10.1016/j.jmb.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 57.Yohannan S, Faham S, Yang D, Whitelegge JP, Bowie JU. The evolution of transmembrane helix kinks and the structural diversity of G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2004;101:959–963. doi: 10.1073/pnas.0306077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buurman ET, McLaggan D, Naprstek J, Epstein W. Multiple paths for nonphysiological transport of K+ in Escherichia coli. J. Bacteriol. 2004;186:4238–4245. doi: 10.1128/JB.186.13.4238-4245.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guffanti AA, Wei Y, Rood SV, Krulwich TA. An antiport mechanism for a member of the cation diffusion facilitator family: divalent cations efflux in exchange for K+ and H+ Mol. Microbiol. 2002;45:145–153. doi: 10.1046/j.1365-2958.2002.02998.x. [DOI] [PubMed] [Google Scholar]
- 60.Southworth TW, Guffanti AA, Moir A, Krulwich TA. GerN, an endospore germination protein of Bacillus cereus, is an Na+/H+-K+ antiporter. J. Bacteriol. 2001;183:5896–5903. doi: 10.1128/JB.183.20.5896-5903.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Truong-Bolduc QC, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J. Bacteriol. 2005;187:2395–2405. doi: 10.1128/JB.187.7.2395-2405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]