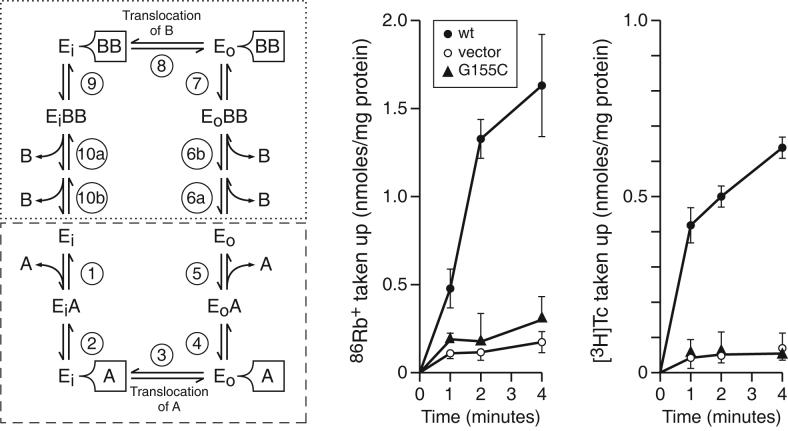
Figure. 3.
Assays of [3H]Tc-Co2+:Tc-Co2+ and K+-86Rb+: K+-Rb+ exchange in RSO vesicles expressing Tet(L) G155C. Left: A diagram of the reaction cycle for Tet(L): Ei and Eo respectively represent the inward and outward facing conformations of the transporter; A is the efflux substrate (e.g. Tc-Co2+) and B is a coupling ion which could be either H+ or Rb+-K+. The B:A ratio is greater than unity (42); the precise stoichiometry is not known and is shown here as 2B:A for diagrammatic purposes. The reaction cycle illustrates an alternating access or ping-pong model in which efflux substrate A binds to Ei in step 1 followed by hypothetical tight binding (step 2) that may be detected in the G155C mutant because of a defect in subsequent translocation (step 3) that usually occurs immediately after tight binding. This is followed by hypothesized release from tight binding (step 4) and final substrate release (step 5). The Tc:Co2+ exchange reaction assesses this set of reactions (steps 1-5), starting with unlabeled Tc:Co2+ inside and labeled Tc:Co2+ outside and proceeding forward and reverse. A comparable half reaction, going in both directions, with the top half cycle (reactions 6-10, dotted box) is assessed by the K+-86Rb+: K+-Rb+ exchange. Middle and Right: the experimental data for the two exchange reactions. The assays were carried out as described under Materials and Methods in comparison with wild type Tet(L) and control membranes. The data are the averages from two independent preparations and are shown with standard deviations.