Table 2.
Substrate Scope of the Tandem Cyclization
| Entry | Substrate | product(s) | Yield(%)a |
|---|---|---|---|
| 1b |
2 |
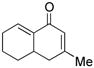
4 |
80 |
| 2b |
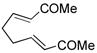
8 |
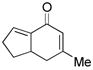
9 |
76 |
| 3b |
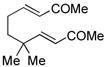
10 |
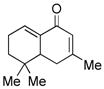
11 |
71 |
| 4c |
12 |
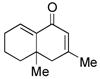
13 |
60d |
| 5c |
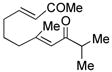
14 |
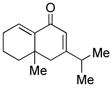
15 |
64d |
| 6c |
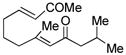
16 |
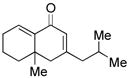
17 |
58d |
| 7c |
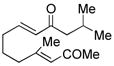
18 |
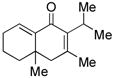
19 |
58d |
The only product isomers detected by 1H NMR analysis of the crude material are those indicated.
Method A: 1 equiv PMe3, 0.05 M substrate in CF3CH2OH, 60 °C.
Method B: 5 equiv PMe3, 0.05 M substrate in t- AmylOH, 80 °C.
The MBH intermediate (c.f., 1) was isolated in 7–8% yield.
