Abstract
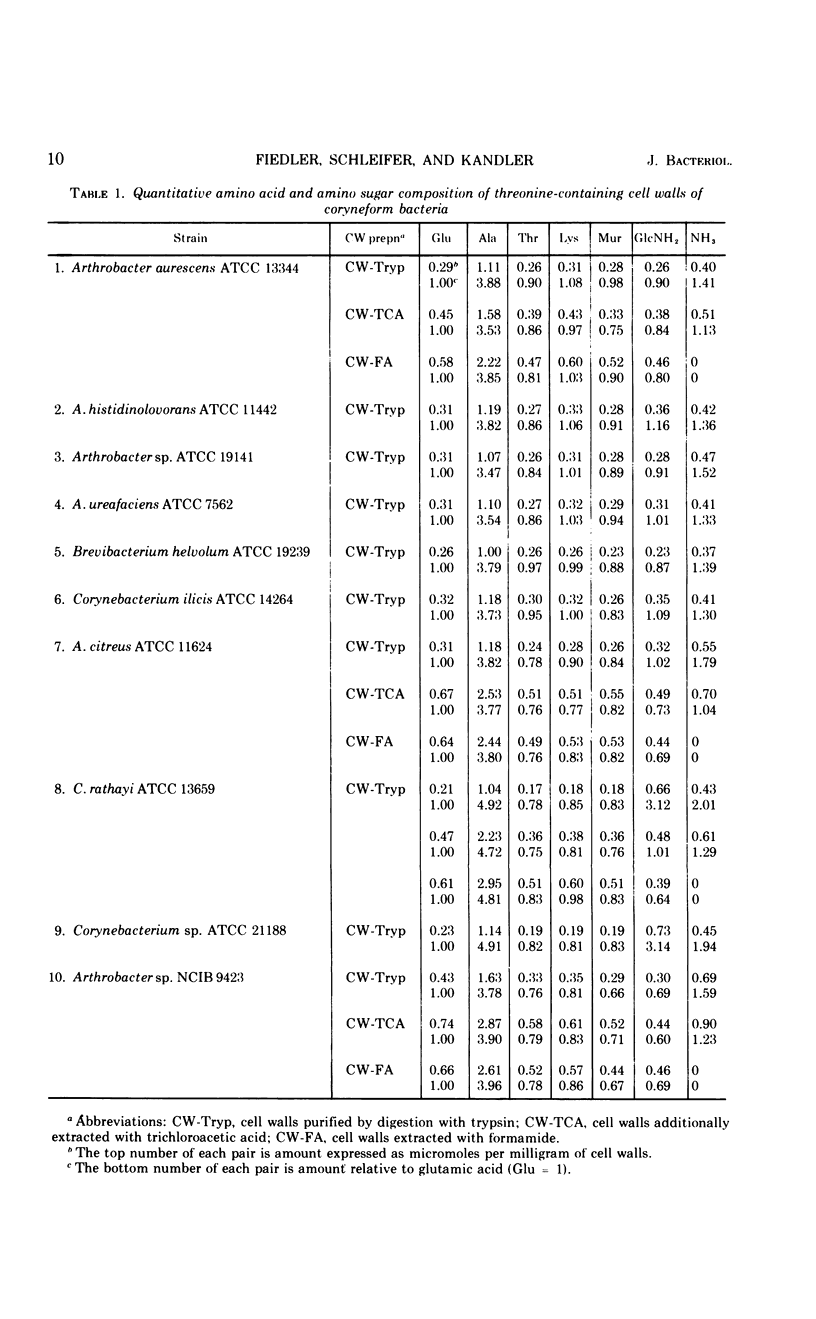
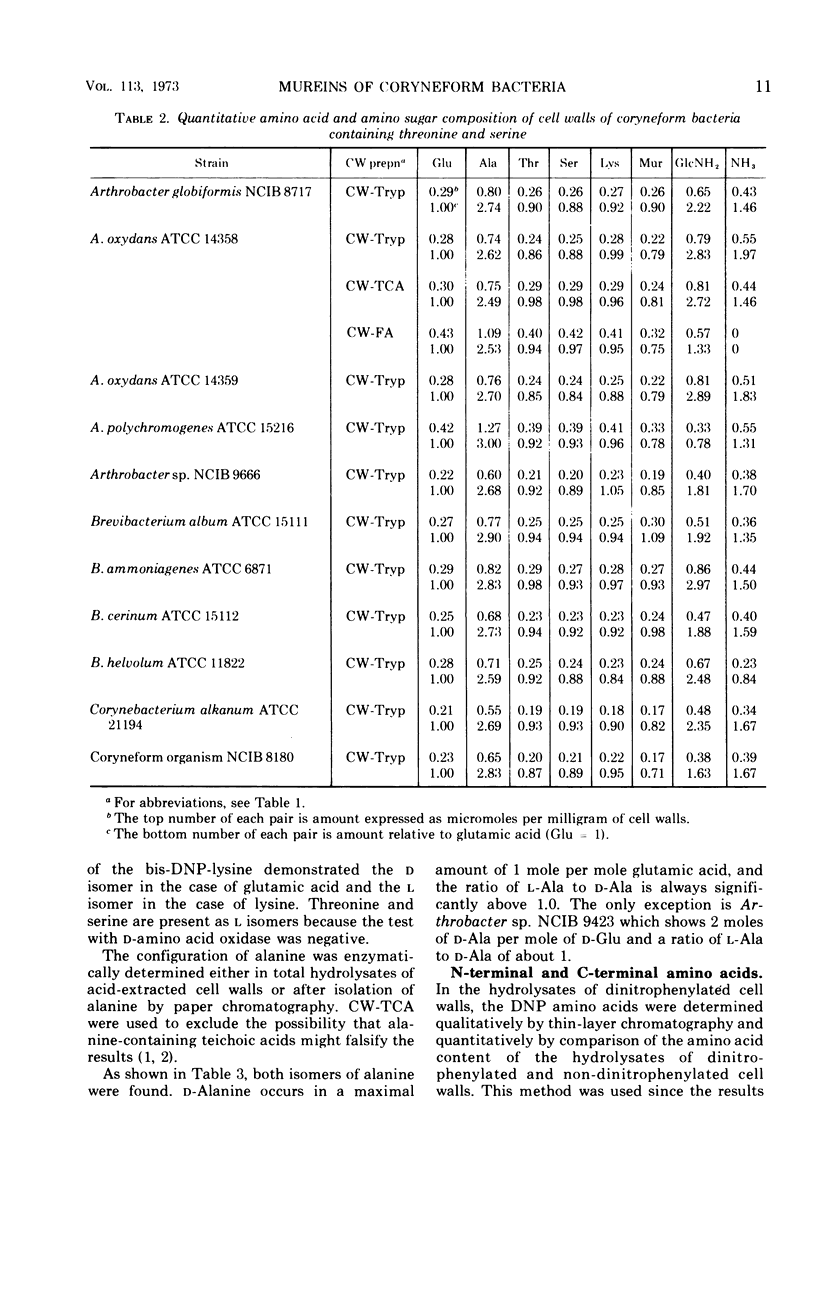
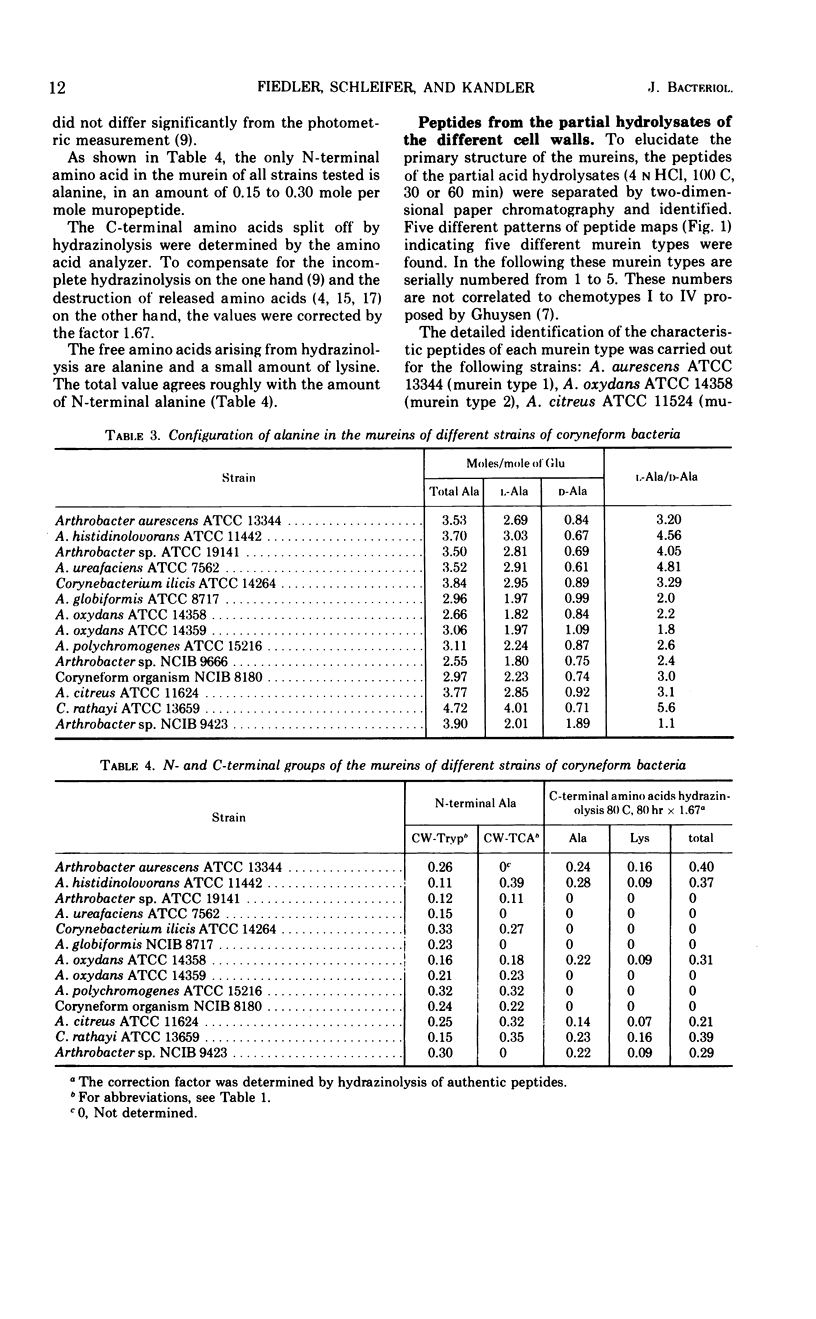
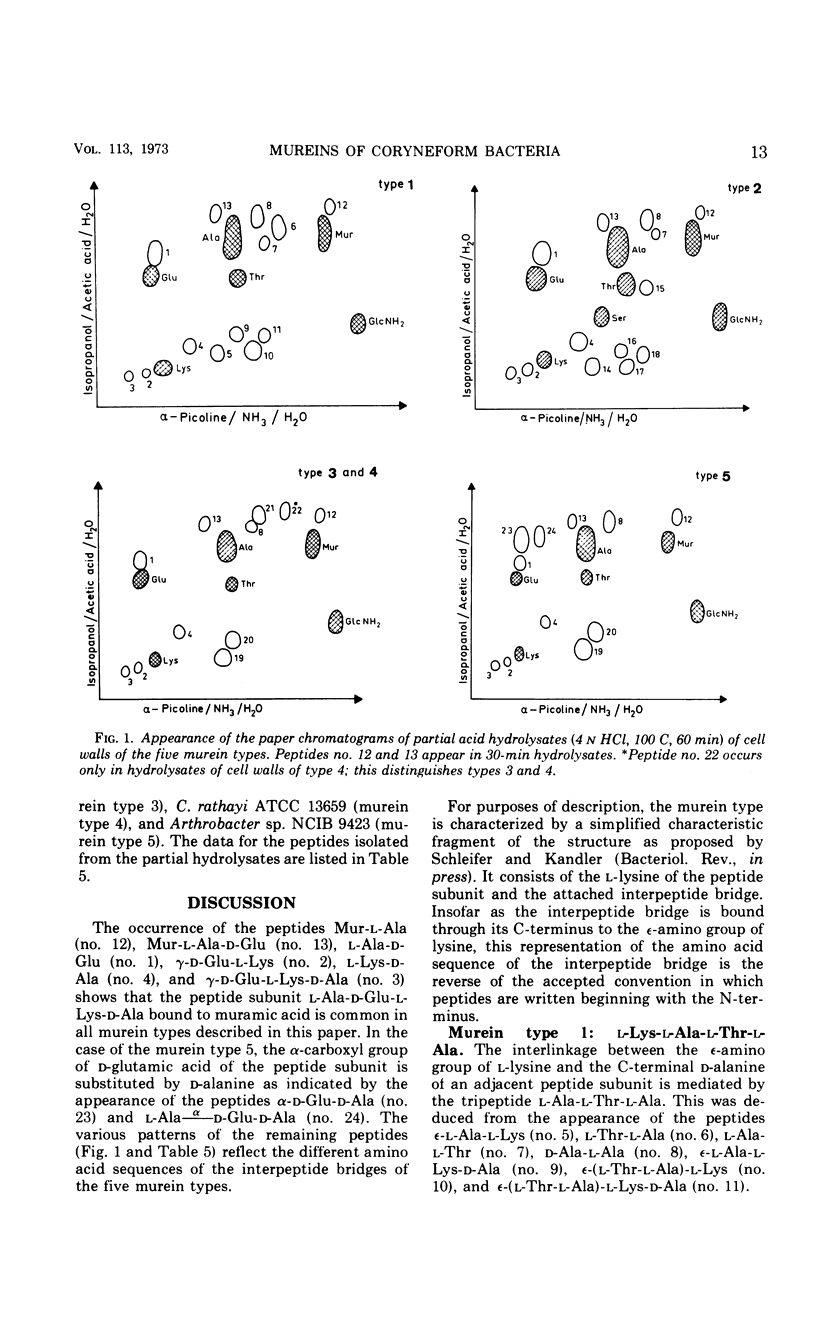
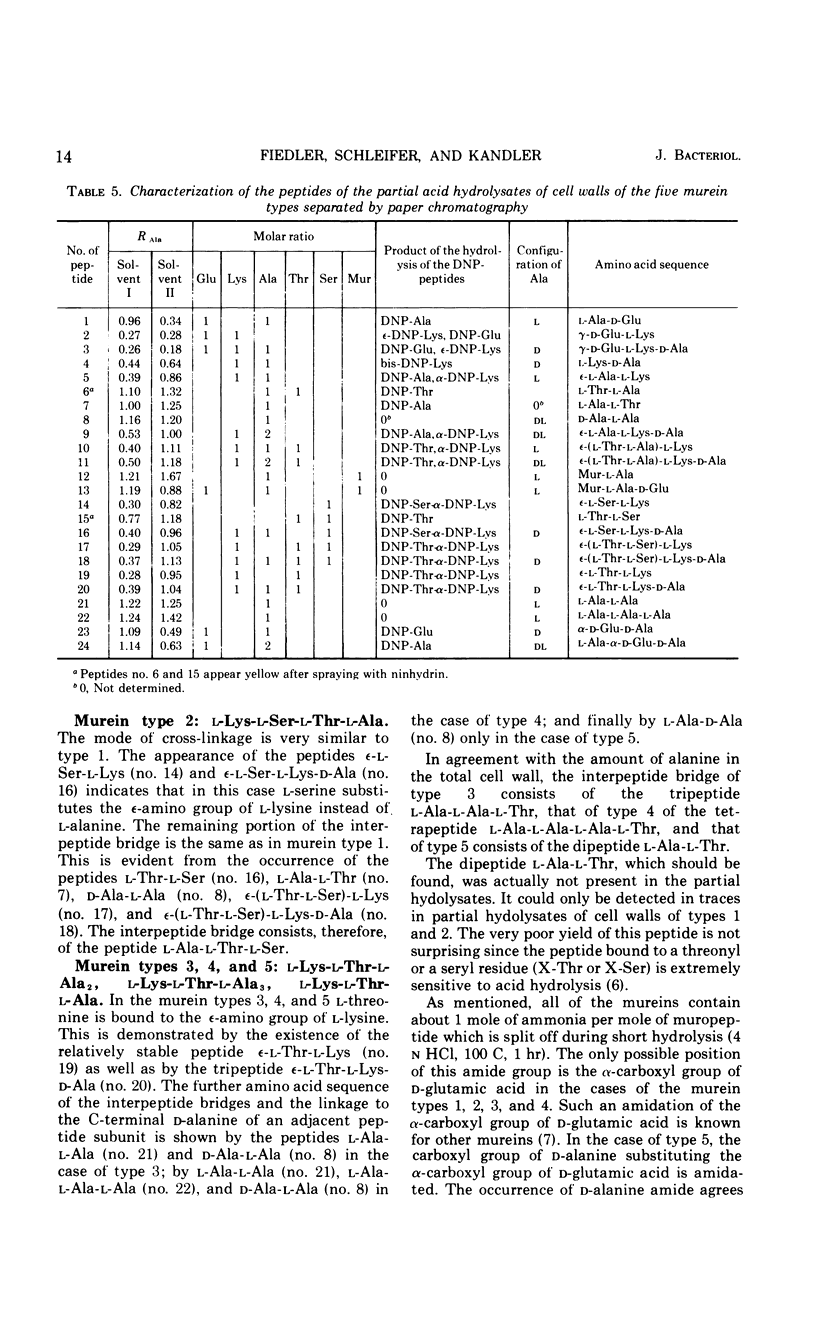
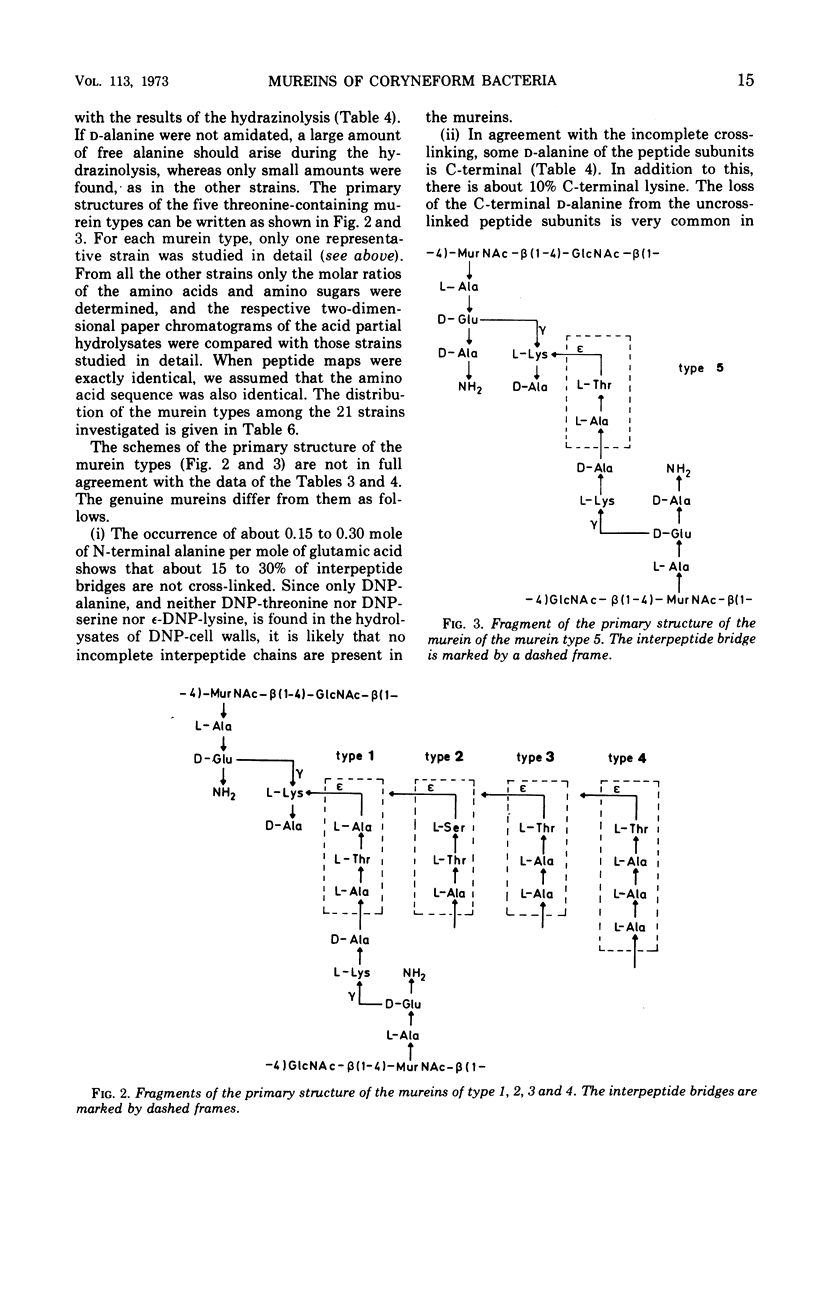
In a study of the mureins of coryneform bacteria (Arthrobacter, Brevibacterium, Cellulomonas, Corynebacterium, Erysipelothrix), 21 threonine-containing strains were found. In several of the strains the amino acid and amino sugar composition of the murein was muramic acid (Mur), glucosamine (GlcNH2), d-Glu, l-Lys, l-Thr, and Ala in a molar ratio of 1:1:1:1:1:4 or 5, and in several other strains it was Mur, GlcNH2, d-Glu, l-Lys, l-Thr, Ala, and l-Ser in a molar ratio of 1:1:1:1:1:3:1. The amino acid sequence of the mureins was determined by analyzing the oligopeptides derived from partial acid hydrolysates. It was shown that there were five different murein types. The peptide subunits attached to the muramic acid are the same, namely l-Ala-d-GluNH2-l-Lys-d-Ala. In one strain, the α-carboxyl group of d-Glu is substituted by d-alanine amide. The interpeptide bridges of the different types consist of the peptides l-Ala-l-Thr-l-Ala, l-Ala-l-Thr, l-Ala-l-Ala-l-Thr, l-Ala-l-Ala-l-Ala-l-Thr, or l-Ala-l-Thr-l-Ser which are bound through their C-termini (l-Ala, l-Thr, l-Ser) to the ε-amino group of l-Lys of one peptide subunit and by their N-termini (l-Ala) to the C-terminal d-Ala of an adjacent peptide subunit. Determination of the N- and C-terminal groups in the mureins showed that about 15 to 30% of the interpeptide bridges are not cross-linked.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. J., BADDILEY J., BUCHANAN J. G., DAVISION A. L., KELEMEN M. V., NEUHAUS F. C. Composition of teichoic acids from a number of bacterial walls. Nature. 1959 Jul 25;184:247–248. doi: 10.1038/184247a0. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG J. J., BADDILEY J., BUCHANAN J. G. Structure of the ribitol teichoic acid from the walls of Bacillus subtilis. Biochem J. 1960 Sep;76:610–621. doi: 10.1042/bj0760610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn H. J., Dimmick I. Soil Bacteria Similar in Morphology to Mycobacterium and Corynebacterium. J Bacteriol. 1947 Sep;54(3):291–303. doi: 10.1128/jb.54.3.291-303.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- IKAWA M., SNELL E. E. Cell wall composition of lactic acid bacteria. J Biol Chem. 1960 May;235:1376–1382. [PubMed] [Google Scholar]
- Kane J., Lackland H., Karakawa W. W., Krause R. M. Chemical studies on the structure of mucopeptide isolated from Streptococcus bovis. J Bacteriol. 1969 Jul;99(1):175–179. doi: 10.1128/jb.99.1.175-179.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D. M., Snetsinger D. C., Waibel P. E. Procedure for determination of D-amino acids. Anal Biochem. 1971 Feb;39(2):395–401. doi: 10.1016/0003-2697(71)90429-5. [DOI] [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- Petit J. F., Munoz E., Ghuysen J. M. Peptide cross-links in bacterial cell wall peptidoglycans studied with specific endopeptidases from Streptomyces albus G. Biochemistry. 1966 Aug;5(8):2764–2776. doi: 10.1021/bi00872a037. [DOI] [PubMed] [Google Scholar]
- Phillips D. M. Loss of C-terminal amino acids by hydrazidation during hydrazinolysis. J Chromatogr. 1968 Sep 24;37(1):132–133. doi: 10.1016/s0021-9673(01)99087-9. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. VIII. Reaction of walls with hydrazine and with fluorodinitrobenzene. Biochim Biophys Acta. 1961 Sep 16;52:329–342. doi: 10.1016/0006-3002(61)90682-5. [DOI] [PubMed] [Google Scholar]
- SWALLOW D. L., ABRAHAM E. P. Formation of epsilon-(aminosuccinyl)-lysine from epsilon-aspartyl-lysine from bacitracin A, and from the cell of lactobacilli. Biochem J. 1958 Nov;70(3):364–373. doi: 10.1042/bj0700364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H. Die Murein (Pepidoglycan)-typen bei grampositiven Bakterien. Zentralbl Bakteriol Orig. 1970;212(2):443–451. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Zur chemischen Zusammensetzung der Zellwand der Streptokokken. I. Die Aminosäuresequenz des Mureins von Str. thermophilus und Str. faecalis. Arch Mikrobiol. 1967 Jul 6;57(4):335–364. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Zur chemischen Zusammensetzung der Zellwand der Streptokokken. II. Die Aminosäuresequenz des Mureins von Str. lactis und cremoris. Arch Mikrobiol. 1967 Jul 6;57(4):365–381. [PubMed] [Google Scholar]


