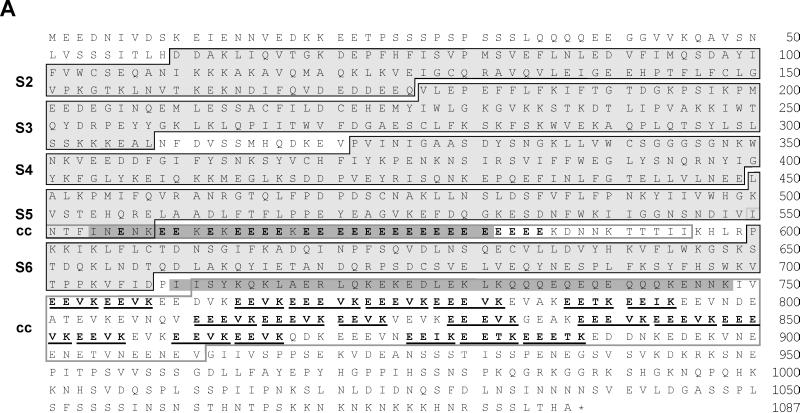
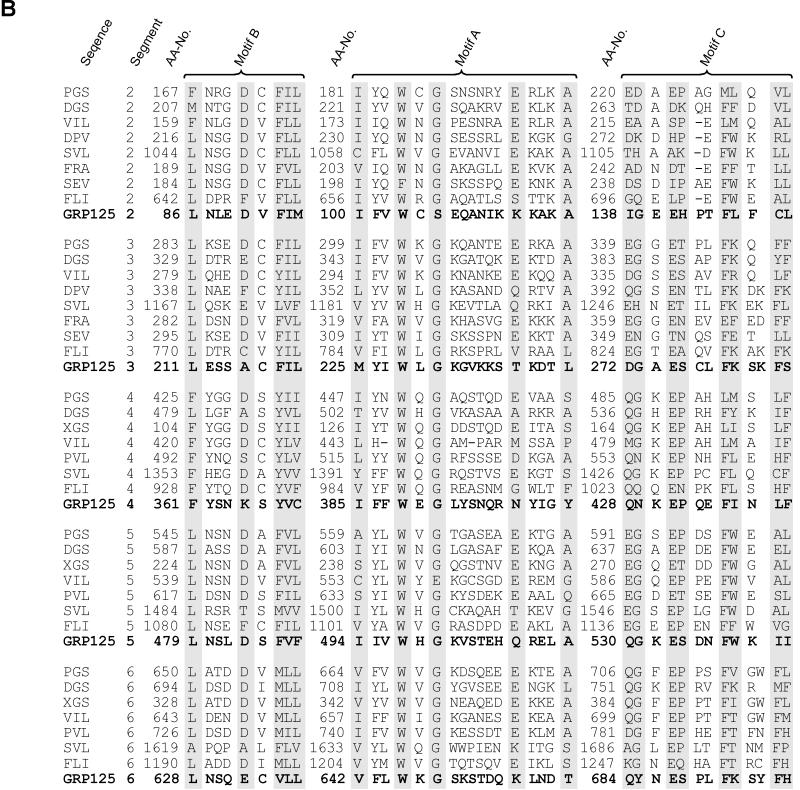
Figure 2.
(A) GRP125’s gelsolin-like segments (S) and repetitive glutamate-rich regions. Black frames indicate segment borders (Way and Weeds, 1988; Eichinger and Schleicher, 1992; Hofmann, 1994). Glutamate-rich stretches are highlighted in bold; the (E)EEV[I,T]K motifs are underlined. Gray shading is applied to coiled-coil regions (cc) predicted with weighting of heptad positions a and d. Gray frames outline coiled-coil regions according to predictions without weighting options. (B) Alignment of motifs (see A) conserved in all domains of gelsolin family members. DGS, Drosophila gelsolin (Heintzelmann et al., 1993); PGS, pig plasma gelsolin (Way and Weeds, 1988); VIL, chicken villin (Bazari et al., 1988); DPV, Dictyostelium protovillin (Hofmann et al., 1993); SVL, bovine supervillin (Pestonjamasp et al., 1997); FRA, fragmin from Physarum (Ampe et al., 1987); SEV, severin from Dictyostelium (Andréet al., 1988); FLI, human flightless I homologue (Campbell et al., 1993); aa-no., amino acid number (modified from Bazari et al., 1988; Way and Weeds, 1988; Heintzelmann et al., 1993). (C) Presence and conservation of motifs at the S1–S2 border in gelsolin-related proteins involved in PIP2 binding (Yu et al., 1992), severing of actin filaments (Kwiatkowski et al., 1989), and F-actin binding (Sun et al., 1994). h-pgs, human plasma gelsolin (Kwiatkowski et al., 1986); h-vil, human villin (Arpin et al., 1988); d-pvl, protovillin from Dictyostelium (Hofmann et al., 1993).