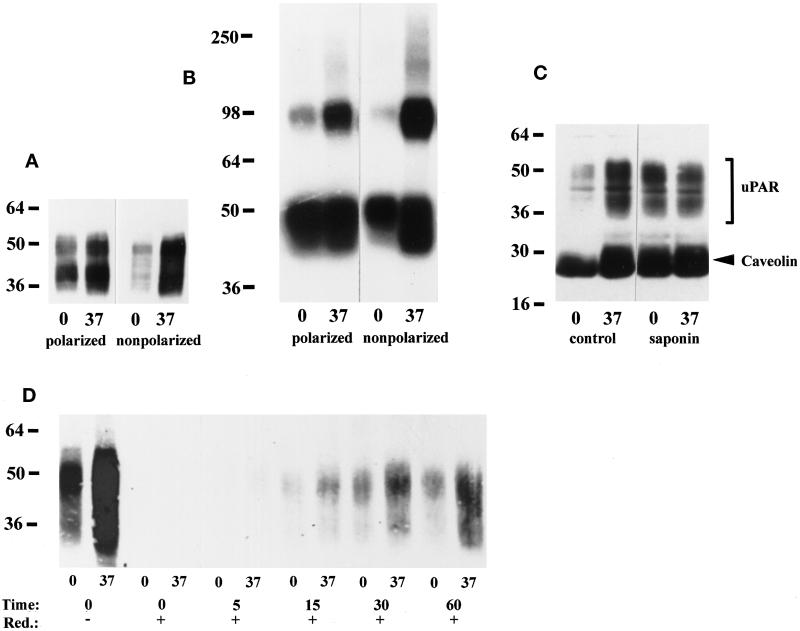
Figure 7.
The fraction of detergent-soluble uPAR is lower in nonpolarized than polarized MDCK cells. (A) Polarized or nonpolarized MDCK cells were extracted with 1% TX-100 (pH 7.4) on ice or at 37°C as indicated. Equal volumes of extract were separated by 10% SDS-PAGE, immobilized on nitrocellulose membranes, and Western blotted with uPAR antibodies as described in the legend to Figure 1A. The blot shown is representative of five experiments. (B) Polarized or nonpolarized MDCK cells were preincubated with 1 nM DFP-uPA at 4°C before chemical cross-linking with 1 mM DSS. TX-100 extracts were then prepared as described above and resolved by 10% SDS-PAGE. Dried gels were autoradiographed for 48 h. Cross-linked uPAR migrating at ∼95 kDa and free DFP-uPA present at the bottom of the gel are evident. (C) Polarized MDCK cells were extracted with 1% TX-100 (pH 6.5) on ice or at 37°C without (−) or with (+) 0.2% saponin, before 4–20% SDS-PAGE and transfer to nictrocellulose membranes, which were probed with a mixture of polyclonal antibodies to uPAR (bracket) and caveolin (arrowhead). (D) Nonpolarized MDCK cells were surface biotinylated with NHS-SS-biotin on ice and then chased at 37°C for the indicated times before reduction with a membrane-impermeable reducing agent on ice. Control dishes were kept on ice throughout and were reduced (t = 0, +) or not (t = 0, −). TX-100 extraction was performed as in A, and uPAR was immunoprecipitated with rabbit antibodies before SDS-PAGE and Western blotting with HRP-conjugated streptavidin.