Figure 8.
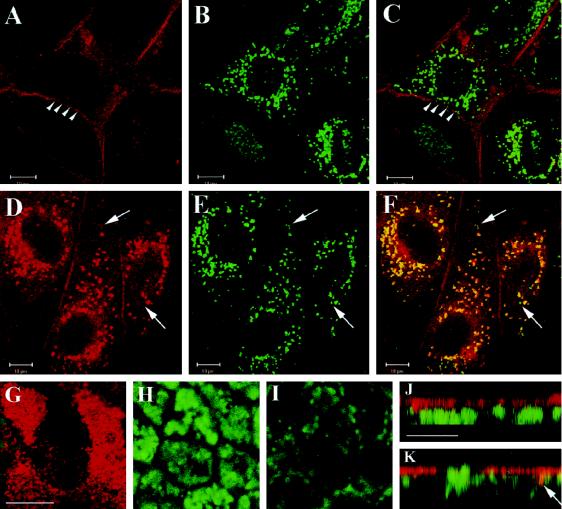
uPA:PAI-1 but not DFP-uPA causes accumulation of uPAR in lysosomes of nonpolarized MDCK cells. Nonpolarized (A–F) or filter-grown, polarized (G–K) MDCK cells were incubated with 100 nM DFP-uPA (A–C) or uPA:PAI-1 (D–K) in normal growth medium containing lysosomal protease inhibitors for 18 h with one intermittent change of incubation medium. Cells were processed for double-label immunofluorescence with anti-uPAR monoclonal antibody R2 and polyclonal anti-LAMP-1 antibodies, respectively, followed by Texas Red–conjugated goat anti-mouse or FITC-conjugated goat anti-rabbit antibodies as appropriate. Images were acquired by confocal microscopy. Shown are the seperate channels for uPAR labeling (A and D) and LAMP-1 labeling (B and E) and merged channels (C, F, and G–K). In nonpolarized cells, a single focal plane through the center of the cells shows that uPA:PAI-1 (D–F), but not DFP-uPA (A–C), caused uPAR to colocalize with LAMP-1 in perinuclear lysosomes. Arrowheads in A and C point to uPAR labeling of lateral membranes; arrows in F point to lysosomes not labeled for uPAR. (G–I) Merged channels of focal planes through apical (G), medial (H), and bottom (I) sections of the polarized monolayer. uPAR expression in monolayers was often heterogenous, as seen in G. uPAR is confined to the apical region (note typical microvillar labeling in G), well separated from LAMP-1–positive intracellular compartments. This is also evident in J and K, which show representative x–z confocal views compiled from stacks of 12 integration frames. Some areas display slight colocalization (K, arrow), but mostly uPAR and LAMP-1 were spatially separated. Bars, 10 μm.