Fig. 4.
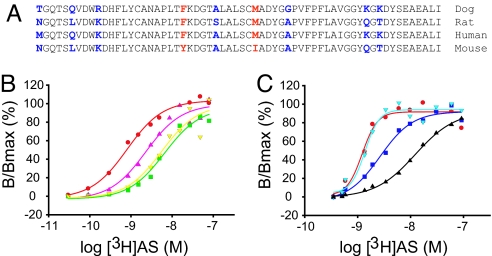
Two residues in loop C are critical for high-affinity [3H]AS binding to NPC1L1. (A) Sequence alignment of a 61-aa subdivision across multiple species of NPC1L1. The amino acid sequence of NPC1L1 (residues 510–571) corresponding to high-affinity (dog, rat, and human) or low-affinity (mouse) [3H]AS binding is shown. Nonconserved positions for which there is a correlation between ligand binding affinity and amino acid identity are highlighted in red, whereas those nonconserved positions where there is no correlation between ligand-binding affinity and amino acid identity are highlighted in blue. (B) Gain of [3H]AS-binding NPC1L1 mutants. TsA-201 cells were transfected with dog NPC1L1 (red circle) or mouse NPC1L1 with the mutations Tyr-532→Phe (green square), Ile-543→Met (yellow inverted triangle), or Tyr-532→Phe/Ile-543→Met (magenta triangle) and incubated with increasing concentrations of [3H]AS, as indicated in Experimental Procedures. Specific binding data were fit to a single class of [3H]AS-binding sites and are presented relative to the maximum receptor occupancy; dog NPC1L1 [(red circle) Kd 0.81 nM, Bmax 3,878 cpm], Tyr-532→Phe [(green square) Kd 6.6 nM, Bmax 7,873 cpm], Ile-543→Met [(yellow inverted triangle) Kd 5.6 nM, Bmax 11,313 cpm], and Tyr-532→Phe/Ile-543→Met [(magenta triangle) Kd 2.29 nM, Bmax 12,701 cpm]. (C) Loss of [3H]AS-binding NPC1L1 mutants. TsA-201 cells were seeded and transfected with dog NPC1L1 (red circle) or dog NPC1L1 with the mutations Phe-532→Tyr (dark blue square), Met-543→Ile (cyan inverted triangle), or Phe-532→Tyr/Met-543→Ile (black triangle) and incubated with increasing concentrations of [3H]AS, as indicated in Experimental Procedures. Specific binding data were fit to a single class of [3H]AS-binding sites and are presented relative to the maximum receptor occupancy; dog NPC1L1 [(red circle) Kd 1.23 nM, Bmax 11,445 cpm], Phe-532→Tyr [(dark blue square) Kd 2.69 nM, Bmax 8,101 cpm], Met-543→Ile [(cyan inverted triangle) Kd 1.38 nM, Bmax 8,258 cpm] and Phe-532→Tyr/Met-543→Ile [(black triangle) Kd 12.4 nM, Bmax 7,940 cpm].