Abstract
When activity levels are altered over days, a network of cells is capable of recognizing this perturbation and triggering several distinct compensatory changes that should help to recover and maintain the original activity levels homeostatically. One feature commonly observed after activity blockade has been a compensatory increase in excitatory quantal amplitude. The sensing machinery that detects altered activity levels is a central focus of the field currently, but thus far it has been elusive. The vast majority of studies that reduce network activity also reduce neurotransmission. We address the possibility that reduced neurotransmission can trigger increases in quantal amplitude. In this work, we blocked glutamatergic or GABAA transmission in ovo for 2 days while maintaining relatively normal network activity. We found that reducing GABAA transmission triggered compensatory increases in both GABA and AMPA quantal amplitude in embryonic spinal motoneurons. Glutamatergic blockade had no effect on quantal amplitude. Therefore, GABA binding to the GABAA receptor appears to be a critical step in the sensing machinery for homeostatic synaptic plasticity. The findings suggest that homeostatic increases in quantal amplitude may normally be triggered by reduced levels of activity, which are sensed in the developing spinal cord by GABA, via the GABAA receptor. Therefore, GABA appears to be serving as a proxy for activity levels.
Keywords: activity, neurotransmitter receptor, postsynaptic, synaptic scaling, synaptic plasticity
Spontaneous network activity (SNA) is a prominent feature of developing neural networks that consists of episodic bursts of activity separated by periods of quiescence (1–3). SNA is produced by hyperexcitable, recurrently connected circuits in which glutamate and GABA are both excitatory early in development. In the spinal cord, SNA drives embryonic limb movements and is known to be important for various aspects of limb (4) and motoneuron development (5, 6). Reducing spontaneous network activity in the embryonic chick in vivo leads to compensatory increases in the strength of excitatory GABAergic and AMPAergic synapses (7). These compensatory increases in synaptic strength have been described in several systems where they appear to act in a manner to recover and maintain network activity levels homeostatically (homeostatic synaptic plasticity) (8, 9).
Activity perturbations lead to several different forms of homeostatic synaptic plasticity, including changes in quantal amplitude, probability of release, and frequency of miniature postsynaptic currents (mPSCs). In the present work, we focus on compensatory changes in quantal (mPSC) amplitude. Changes in mPSC amplitude have been studied intensely in cultured networks where prolonged activity reduction results in an increase in the amplitude of excitatory mPSCs and a decrease in the amplitude of inhibitory mPSCs (10–12). Changes in mPSC amplitude are achieved through alterations in the number of postsynaptic receptors and/or the amount of transmitter released per vesicle (8, 9). However, far less is known about the machinery that senses perturbations in the level of activity, which then trigger compensatory changes in mPSC amplitude.
Several signals have been suggested as possible sensors for tracking changes in activity, including spike rate, depolarization, and intracellular calcium levels (8, 9, 13–15). One of the favored models suggests that reducing network activity produces a corresponding reduction in cellular spiking activity, thereby reducing intracellular calcium levels in a postsynaptic cell. In this model, which we will refer to as the cell activity model, the postsynaptic cell senses changes in intracellular calcium levels as a measure of altered activity and triggers compensatory changes in mPSC amplitude. However, the studies that have inspired this model not only block spiking activity, they also block or reduce neurotransmitter binding to its receptor and any associated downstream signaling cascades. Thus, it remains possible that neurotransmission is a critical step in the sensing process that triggers changes in quantal amplitude. In an alternative model, the neurotransmitter model, changes in activity levels would be sensed by alterations in neurotransmitter receptor binding. To address such a possibility, one would need to block either activity or neurotransmission, but not both. Recent studies have demonstrated that neither hyperpolarization nor reducing spike rate in the postsynaptic cell leads to compensatory changes in mPSC amplitude as would be predicted by the cell activity model (8, 16–19). In those studies, the release and binding of neurotransmitters to their receptors was not altered, and thus the results are consistent with the neurotransmitter model.
To test the neurotransmitter model directly, one would like to block neurotransmission without affecting activity levels. In the present work, we block GABAergic or glutamatergic neurotransmission in the developing spinal cord in vivo while maintaining significant levels of SNA caused by the fast compensatory nature of the highly excitable developing cord (20, 21). We have found a particular importance of GABAA transmission in triggering compensatory changes in quantal amplitude. Our findings suggest that the GABAA receptor is part of the sensing machinery for homeostatic synaptic plasticity in the developing spinal cord.
Results
Neurotransmitter Block in Ovo.
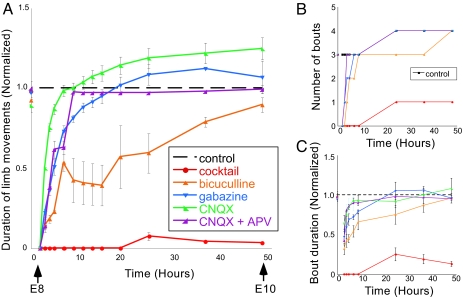
Previously, we reduced SNA in ovo from embryonic day 8–10 (E8–E10) through infusion of the sodium channel blocker lidocaine and found compensatory increases in both GABAergic and AMPAergic mPSC amplitude at E10 (7). To test the possibility that reduced ionotropic neurotransmitter signaling triggered compensatory changes in quantal amplitude, we injected various neurotransmitter antagonists in ovo. We first determined the concentration of receptor antagonists necessary to reduce neurotransmission significantly for 48 h in ovo. Embryonic limb movements were assessed to determine the effects of neurotransmitter receptor block on spontaneous network activity in ovo. Spinally generated embryonic limb movements were observed through windows in the eggshell. SNA was quantified in ovo by counting the total time the chicken embryo moved during a 5-min period of observation (Fig. 1A). We also monitored the number of bouts of movement (Fig. 1B) and the duration of each bout (Fig. 1C).
Fig. 1.
GABAergic or glutamatergic receptors can be chronically blocked without a prolonged reduction of spontaneous network activity. (A) Graph of the average amount of time chick embryos moved during a 5-min period of observation, obtained once every 0.5–12 h. Data are normalized to control values at each observation time point/stage, thus producing the dotted line for comparison. Treatment with a single receptor antagonist (bicuculline, n = 4 embryos; gabazine, n = 5; CNQX, n = 3; CNQX + APV, n = 6) transiently reduces embryonic movements; however, movements return to control (n = 13) levels within 12 h. A mixture of gabazine, CNQX, strychnine, and APV (n = 9) nearly abolished limb movements for 48 h. (B and C) Graph of the median number of bouts or episodes (B) or average bout duration (C) during a 5-min period of observation. Error bars, SE.
To determine the effective concentrations of receptor antagonists needed to block activity significantly in ovo, a mixture of ionotropic receptor antagonists was infused into the egg, including a GABAA receptor antagonist (bicuculline or gabazine, concentrations shown in Experimental Procedures; assume a 50 ml egg volume), a glycine receptor antagonist (strychnine), an AMPA/KA (referred to as AMPA from this point) receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), and an NMDA receptor antagonist DL-2-amino-5-phosphonopentanoic acid (APV). The injection of the mixture of these four antagonists at E8 resulted in a near cessation of all limb movements from E8 to E10 (Fig. 1, red).
The exclusion of any one of these drugs from the mixture reduced limb movements but did not abolish them for the entire 2 days (data not shown). The injection of a single receptor antagonist or a combination of CNQX and APV caused a temporary reduction in limb movements that recovered to control levels within hours (Fig. 1). Doubling the concentration of any drug or the addition of a second bolus at E9 produced no additional effect on limb movements (data not shown). This finding suggested that the drugs were still effective but that the spinal networks controlling limb movements had compensated for the loss of transmission (22). These experiments demonstrate that GABAA or AMPA/NMDA receptor activation can be significantly reduced in ovo from E8 to E10, whereas levels of SNA remained largely intact, apart from a transient reduction after drug administration.
No Change in mPSCs After AMPA/NMDA Receptor Block.
To test the consequences of blocking ionotropic glutamatergic receptor signaling, we obtained whole-cell voltage clamp recordings (holding potential, −70 mV) from antidromically identified motoneurons of embryos treated from E8 to E10 with saline (1 ml), CNQX alone, or CNQX and APV. Recordings of mPSCs were obtained in the absence of receptor antagonists unless otherwise indicated. GABA and AMPA mPSCs were isolated by their decay kinetics as described in ref. 7 and in Experimental Procedures.
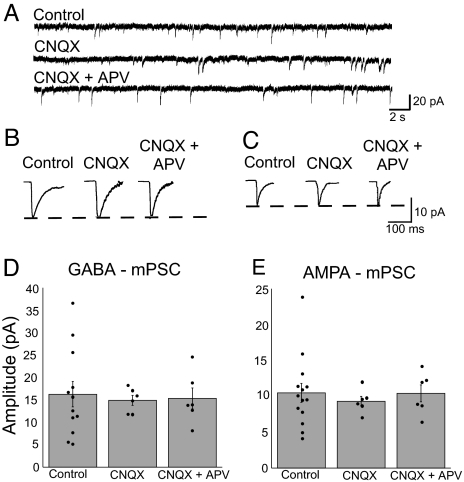
Chronic CNQX or CNQX + APV treatment did not result in a change in the amplitude of AMPA or GABAA mPSCs (Fig. 2 and Table 1). Further, average mPSC frequency, mPSC rise and decay kinetics, and cellular passive membrane properties were unaltered after glutamate receptor blockade from E8 to E10 (Tables 1 and 2). We did see a reduction in the variability of mPSC amplitude when embryos were treated with the glutamate antagonists. Although we do not know what normally produces this variability, it has been suggested that networks maintain a level or pattern of activity with different combinations of distinct parameters, each parameter exhibiting considerable variability (23). If a particular conductance (synaptic receptor/potassium channel) or the probability of release is influenced by antagonist treatment, then it may be that the remaining parameters have a more restricted parameter space and therefore exhibit less variability.
Fig. 2.
Forty-eight hours of ionotropic glutamate receptor blockade did not alter the amplitude of GABA or AMPA mPSCs. (A) Representative voltage clamp recordings from motoneurons of saline-, CNQX-, or CNQX + APV-treated embryos. (B and C) Average GABA mPSC (B) or AMPA mPSC (C) from treated embryos. (D and E) Treatment with CNQX or CNQX + APV from E8 to E10 did not significantly alter the average amplitude of GABA mPSCs (D) or AMPA mPSCs (E) compared with control. Filled circles represent individual data points. Error bars, SE.
Table 1.
mPSC amplitudes and frequencies
| Treatment condition | n | Amplitude, pA | Frequency, Hz | Rise time, ms | Decay time, ms |
|---|---|---|---|---|---|
| GABA mPSC | |||||
| Control | 14 | 16.3 ± 2.8 | 0.3 ± 0.1 | 12.4 ± 5.3 | 50.1 ± 6.4 |
| 48 h bicuculline | 5 | 33.9 ± 5.2* | 0.6 ± 0.2 | 11.6 ± 1.9 | 50.2 ± 5.7 |
| 48 h gabazine | 9 | 29.7 ± 2.6* | 0.3 ± 0.1 | 13.8 ± 5.8 | 53.6 ± 10.7 |
| 12 h gabazine | 7 | 17.5 ± 1.9 | 0.4 ± 0.7 | 12.7 ± 0.8 | 51.2 ± 5.5 |
| 48 h CNQX | 6 | 14.9 ± 1.1 | 0.4 ± 0.9 | 14.3 ± 2.1 | 47.6 ± 3.2 |
| 48 h CNQX + APV | 6 | 15.3 ± 2.3 | 0.3 ± 0.3 | 12.2 ± 1.4 | 41.6 ± 9.5 |
| AMPA mPSC | |||||
| Control | 14 | 10.5 ± 1.3 | 0.8 ± 0.2 | 8.7 ± 5.9 | 9.3 ± 5.3 |
| 48 h bicuculline | 5 | 17.6 ± 2.6† | 1.0 ± 0.4 | 7.5 ± 0.9 | 8.8 ± 1.2 |
| 48 h gabazine | 9 | 17.1 ± 1.3* | 0.6 ± 0.1 | 10.5 ± 6.5 | 7.8 ± 4.6 |
| 12 h gabazine | 7 | 10.4 ± 0.9 | 0.8 ± 0.4 | 11.4 ± 1.7 | 8.5 ± 0.7 |
| 48 h CNQX | 6 | 9.3 ± 0.7 | 0.4 ± 0.1 | 10.7 ± 1.7 | 8.1 ± 1.0 |
| 48 h CNQX + APV | 6 | 10.4 ± 1.2 | 0.4 ± 0.1 | 10.3 ± 1.6 | 8.3 ± 0.9 |
n, number of cells recorded; values are mean ± SE, one-way ANOVA.
*Statistically significant difference compared with control (P ≤ 0.01).
†Statistically significant difference compared with control (P ≤ 0.03).
Table 2.
Passive membrane properties
| Treatment condition | n | Membrane potential, mV | Input resistance, MΩ | Membrane capacitance, pF |
|---|---|---|---|---|
| Control | 14 | −50.6 ± 0.6 | 337.1 ± 31.7 | 21.1 ± 6.8 |
| 48 h bicuculline | 5 | −52.5 ± 1.8 | 298.0 ± 20.4 | 19.8 ± 3.8 |
| 48 h gabazine | 9 | −53.6 ± 2.6 | 266.2 ± 22.1 | 23.6 ± 7.3 |
| 12 h gabazine | 7 | −52.3 ± 1.2 | 307.3 ± 20.3 | 16.4 ± 2.6 |
| 48 h CNQX | 6 | −51.7 ± 0.7 | 278.3 ± 16.9 | 17.8 ± 0.6 |
| 48 h CNQX + APV | 6 | −51.3 ± 0.4 | 277.8 ± 16.8 | 15.7 ± 1.2 |
These findings suggest that reducing glutamatergic transmission did not trigger compensatory changes in quantal amplitude observed after activity blockade. This finding is consistent with the cell activity model or the neurotransmitter model, assuming that GABA is the key neurotransmitter. GABAA signaling has been shown to be particularly important for several aspects of early development (24).
GABAA Receptor Block Increased mPSC Amplitude.
To test the consequences of blocking GABAA receptor signaling, we obtained whole-cell recordings from motoneurons as described above, but from embryos treated with a GABAA receptor antagonist (bicuculline) from E8 to E10. GABAergic mPSC amplitude was significantly greater in bicuculline-treated embryos than in controls (108% increase; Table 1 and Fig. 3). We found that full recovery of embryonic movements did not occur until E10 after bicuculline treatment at E8 (Fig. 1). This recovery may occur because of nonspecific effects of bicuculline (25) or because bicuculline acts at both synaptic and extrasynaptic GABAA receptors. We therefore also treated embryos with gabazine, which is thought to block synaptic GABAA receptors preferentially (26), and we found that embryonic movements recovered more quickly (Fig. 1). GABAergic mPSC amplitudes from gabazine-treated embryos were again significantly greater than controls (82% increase) but were not different from embryos treated with bicuculline (Table 1 and Fig. 3). We also observed that the entire distribution of GABAergic mPSCs increased by a multiplicative factor, or, in other words, the distribution scaled (ref. 12 and Fig. 3F). These data suggest that blocking GABAA receptor transmission leads to an increase in the amplitude of GABAergic mPSCs.
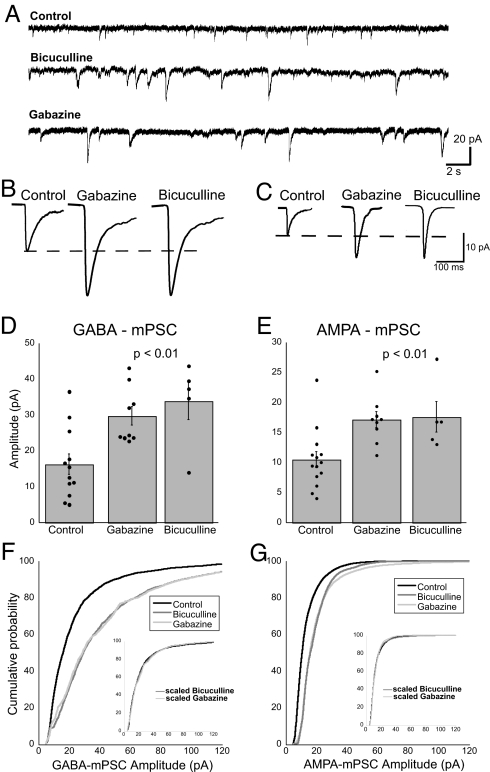
Fig. 3.
Forty-eight hours of GABAA receptor blockade increased the amplitude of GABA and AMPA mPSCs. (A) Representative voltage clamp recordings from motoneurons of saline-, bicuculline-, and gabazine-treated embryos. (B and C) Average GABA mPSC (B) or AMPA mPSC (C) from treated embryos. (D and E) The average amplitude of GABA mPSCs (D) or AMPA mPSCs (E) was significantly increased in bicuculline- and gabazine-treated motoneurons compared with control. Error bars, SE. (F and G) Cumulative histogram shows that the entire distribution of GABA mPSC amplitudes (F) or AMPA mPSC amplitudes (G) for motoneurons from bicuculline- (dark gray) and gabazine-treated (light gray) embryos shifted to the right. The bicuculline and gabazine distributions are similar (P = 0.01, GABAA mPSCs; P = 0.03, AMPA mPSCs). (F Inset) The bicuculline and gabazine distributions could be collapsed onto the control distribution by dividing every point in the distribution by a factor of 2.0 (P = 0.03), suggesting that they scale. Amplitudes <5 pA were excluded from the distributions both before and after scaling the distributions. (G Inset) The bicuculline distributions could be collapsed onto the control distribution by dividing every point in the distribution by a factor of 1.5 (P = 0.03). The gabazine distribution nearly collapsed onto the control distribution after dividing each point by a factor of 1.25 (P = 0.07).
AMPA mPSCs from GABAA receptor-blocked embryos were kinetically isolated from the same set of motoneurons described above. Chronic GABAA receptor antagonist treatment significantly increased the amplitude of AMPA mPSCs compared with controls, even though AMPA receptors were not blocked (63% increase with gabazine, 68% increase with bicuculline; Table 1 and Fig. 3). Again, the entire distribution of AMPAergic mPSCs appeared to scale (Fig. 3G). No significant differences were found in the frequency, decay, or rise times of GABAA or AMPA mPSCs for any treatment condition (Table 1). Nor were significant differences were found in passive membrane properties between the groups (Table 2).
To determine whether increases in mPSC amplitude in gabazine-treated embryos were caused by a new population of mPSCs with a different pharmacology, we isolated the mPSCs by acute bath application of CNQX and/or gabazine. We found that adding 20 μM CNQX reduced the mPSC population with fast-decay kinetics to only 6.3% of that before the antagonist. Similarly, adding 10 μM gabazine reduced the mPSC population with slow-decay kinetics to only 4.1% of that before the antagonist. When both CNQX and gabazine were added to the bath, mPSCs were virtually abolished (n = 3). These results suggest that chronic GABAA receptor blockade did not reveal a new population of mPSCs with a different pharmacology. Additionally, acute application of tetrodotoxin (TTX) did not significantly change the amplitude (19.6 ± 0.2 pA; then after TTX, 18.6 ± 0.7 pA) or frequency (0.79 ± 0.22 Hz; then after TTX, 0.83 ± 0.19 Hz) of mPSCs (n = 3). Results with antagonists or TTX in gabazine-treated embryos are similar to those observed in control embryos (see Experimental Procedures).
Twelve-Hour GABAA Block Did Not Induce Changes in mPSC Amplitude.
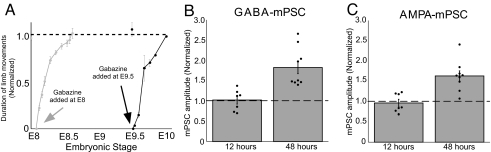
Embryonic movements recover in either GABAA or glutamatergic block within 12 h. One of the hallmarks of homeostatic changes in mPSC amplitude has been that it requires approximately a day of altered activity to develop. We therefore believed that the recovery of embryonic movements occurred through fast transiently existing changes in network excitability that have been described for the isolated spinal cord in vitro (22). Regardless, we wanted to test the possibility that changes in quantal amplitude in the embryonic spinal cord might occur faster than in other systems and contribute to the recovery of embryonic movements. To assess the time course of the increase in mPSC amplitude and to determine whether it could contribute to the recovery of embryonic movements in ovo, we injected gabazine in ovo at E9.5 and assayed quantal amplitude 12 h later at E10. Similar to the findings described above, in ovo limb movements were dramatically reduced and then recovered to near control levels by 12 h of gabazine treatment (Fig. 4A). Recordings obtained from motoneurons of embryos treated for only 12 h with gabazine demonstrated no significant difference in the amplitude of AMPA or GABAA mPSCs (Fig. 4 B and C and Table 1). Neither the frequency of GABA mPSCs nor AMPA mPSCs was significantly different between control and 12 h of GABAA receptor blockade (Table 1). The results suggest that GABAA receptor block must be in place for >12 h to exhibit the increases in mPSC amplitude observed after 48-h treatments. Further, the initial recovery of embryonic movements 12 h after injection of gabazine occurs without the contribution of increases in mPSC amplitude. Finally, in embryos treated from E8 to E10, mPSC amplitude must increase during the period when SNA levels were relatively normal. Together, these results suggest a particular importance for GABAA transmission in triggering homeostatic changes in GABAergic and AMPAergic mPSC amplitudes.
Fig. 4.
Twelve hours of GABAA receptor blockade did not alter the amplitude of GABA or AMPA mPSCs. (A) Graph of the average amount of time chick embryos moved limbs during a 5-min period of observation. Data are normalized to control values at each time point in which observations were collected. Blocking GABAA receptors in ovo (gabazine) for 12 h, beginning at E8 or at E9.5, transiently reduces the embryonic limb movements, which recover within 12 h. (B and C) The average amplitude of GABA mPSCs (B) or AMPA mPSCs (C) is not significantly different among motoneurons from embryos treated with saline (control) or gabazine for 12 h. Gabazine treatment for 48 h significantly increased the amplitude of GABA mPSCs or AMPA mPSCs compared with control (48-h data same as that in Fig. 3 D and E). Dashed lines represent control values. Error bars, SE.
Discussion
In this work we aimed to determine whether neurotransmission was a critical part of the sensing machinery that triggers homeostatic increases in mPSC amplitude after perturbations to network activity levels in the developing spinal cord. These increases could be triggered by reductions in cellular spiking activity [cell activity model (27–29)]. Alternatively, the reduction in SNA could cause a reduction in the release of neurotransmitter, which could trigger compensatory changes in quantal amplitude (neurotransmitter model). In this work we show evidence in vivo supporting the neurotransmitter model rather than the cell activity model. In ovo block of GABAA receptors triggered a strong increase in the amplitude of both GABAA and AMPA mPSCs, even though embryonic movements were largely intact in gabazine-treated embryos. This finding places GABAergic neurotransmission as a critical step in the homeostatic process. Because GABAA receptors are blocked throughout the network, the key site of neurotransmission block that triggers quantal amplitude changes could be at the postsynaptic cell, the presynaptic cell, and/or the resident glia. These findings support the idea that when SNA is reduced, GABAA transmission is also reduced, and this triggers the compensatory increase in quantal amplitude. Therefore, levels of released GABA would be monitored as a proxy for activity.
Embryonic movements are likely to be a reliable indicator of spinal SNA because electromyographic recordings in ovo during embryonic movements are remarkably similar to motoneuron recordings during in vitro SNA (30–33). It is also likely that SNA that recovers after antagonist injections in the egg is similar to SNA before the antagonists were added because similar findings have been observed in the in vitro spinal cord preparation (22). Further, we did not observe differences in several features of the in ovo embryonic movements in treated and control embryos, including total movement duration, bout duration, bout frequency, or recovery of the movements. We remain open to the possibility that subtle distinctions between the embryonic movements and SNA may exist; however, it is unlikely that increases in quantal amplitude are triggered by low activity levels in GABAA-blocked embryos because they exhibit larger increases in mPSC amplitude than activity-blocked lidocaine-treated embryos (7). For this reason it is also highly unlikely that a transient reduction in SNA, after antagonist injection, could account for the observed increase in quantal amplitude. Blocking GABAA receptors for 12 h or blocking glutamate receptors for 48 h produced the same transient reduction and recovery in embryonic movements as 48-h GABAA block but failed to produce the changes in mPSC amplitude observed after 48-h GABA block. These findings suggest that lowered GABAergic neurotransmission triggers compensatory changes in quantal amplitude. The results are consistent with the idea that GABAergic transmission is partly reduced in lidocaine-treated embryos (action potential-dependent release) but is more completely reduced in the presence of GABAA antagonists. Taken together, the findings suggest that chronic blockade of GABAA receptors, rather than a reduction in activity, triggers changes in mPSC amplitude, thereby supporting the neurotransmitter hypothesis.
At E10, the two dominant spinal motoneuron inputs are GABAergic and glutamatergic (7). Blocking AMPA and NMDA receptors failed to trigger changes in the amplitude of mPSCs. Previous studies in cultured networks also have found that NMDA receptor block did not trigger compensatory changes in mPSC amplitude (11, 12). Our findings suggest a special role for GABAergic transmission in triggering the compensatory changes in mPSC amplitude. This places GABA and its receptor early in the hierarchical cascade that leads to downstream changes in both GABAA and AMPA mPSC amplitude. GABA may be capable of triggering changes in quantal amplitude in early development because it is depolarizing and GABAergic synapses may be more fully developed than the AMPAergic system at this stage (34, 35). It is now well appreciated that GABA signaling is involved in directing multiple developmental processes, including cell proliferation, migration, and differentiation; establishment of synaptic connections and their refinement; and potentially in the depolarizing to hyperpolarizing conversion of the GABAA response itself (24, 36–38).
Is it possible that reduced neurotransmission also triggers compensatory changes in mPSC amplitude in other systems where homeostatic synaptic plasticity has been described? In most studies where cultured network activity levels are perturbed, so too are released transmitters; thus, it is possible that altered neurotransmission triggers changes in mPSC amplitude in these studies as well (11, 39, 40). In cultured networks, reducing GABAA transmission triggers a reduction in the amplitude of excitatory mPSC amplitude, which makes functional sense because in these studies GABA is inhibitory, and blocking it increases spiking activity (11, 12, 39). This is opposite to our finding of increased quantal amplitude after GABAA block, although in embryonic spinal neurons, GABA is depolarizing. As a result, GABA may be able to activate downstream signaling cascades early in development because GABAA transmission can lead to calcium entry. Once GABAA transmission is no longer depolarizing, it may not be able to trigger the same downstream cascades, and the role of triggering compensatory quantal changes may be transferred to AMPAergic transmission. In four separate studies (in vitro and in vivo), activity was reduced in only the postsynaptic cell by expression of a potassium channel, whereas neurotransmitter release by the cell inputs remained intact and presumably unaltered (16–19). Although some of these studies reported a compensatory change in probability of release, none of them observed the expected increase in quantal amplitude. Further, in a recent study of homeostatic synaptic plasticity in hippocampal cultured networks, Stellwagen and Malenka (41) show that compensatory increases in AMPAergic mPSC amplitude are triggered by glia that may sense low glutamate levels in the culture medium after reduced activity. Thus, our findings of reduced neurotransmission triggering homeostatic changes in quantal amplitude may be a generally applicable finding for homeostatic synaptic plasticity in conditions of lowered activity. However, a recent study reports that spiking activity is important in triggering a fast form of AMPAergic quantal scaling (≤4 h) that depended on the glial conditions of the culture system (27). It is known that the mechanisms of homeostatic synaptic plasticity after reductions in activity are distinct from those after increased activity (42), and one study suggests that depolarization rather than transmission may be important in triggering compensatory changes in quantal amplitude in response to increased network activity (43).
Embryos treated with CNQX, CNQX + APV, or gabazine recovered normal embryonic movements within 12 h, without changes in quantal amplitude. It is likely that several mechanisms are recruited to recover and maintain activity levels and that the different mechanisms may have different time courses (13). After glutamatergic blockade, activity recovers in part because of a fast loading of intracellular chloride, which strengthens the unblocked GABAergic currents (22, 44). After GABAergic block, there may be fast changes in the probability of release (45) and changes in cellular excitability (46, 47). We have found that after GABAA block in ovo, there are fast changes (≤12 h) in cellular excitability that begin to dissipate toward control levels after 48 h of GABAA block (J.C.W. and P.W., unpublished observations). Therefore, it is possible that SNA recovers after gabazine application through fast changes in cellular excitability and that compensatory changes in mPSC amplitude take longer to be expressed. Similar findings have been reported in activity-blocked cultured networks where changes in current densities of different voltage-gated channels increased the excitability of the neurons (46, 47). The precise details of the compensatory responses underlying the homeostatic recovery of activity levels are not well understood, in part because studies have not directly compared the time course of activity recovery and changes in quantal amplitude. Further, studies tend to focus on one aspect of homeostasis, synaptic or intrinsic excitability. It will be important to consider these compensatory mechanisms together to gain a more complete understanding of the process of homeostatic recovery.
Taken together, our findings suggest that a reduction in GABAA receptor activation triggers compensatory changes in quantal amplitude in the developing spinal cord. The results support the hypothesis that lowered activity is sensed through reduced GABA, via GABAA receptors. This hypothesis would make GABAA receptors a key determinant in the sensing machinery that triggers compensatory changes to maintain network activity in the developing nervous system and would have implications for the role of neurotransmission in homeostatic synaptic plasticity in general.
Experimental Procedures
Dissection.
White Leghorn chicken embryos (Hy-Line Hatcheries) were dissected as described in ref. 48. Briefly, spinal cords from stage 36 (E10) embryos were isolated with intact muscle nerves. The preparation was maintained in a recirculating bath of oxygenated Tyrode's solution, and the dissection was performed at 15°C and left overnight at 17°C. Recordings were performed in oxygenated Tyrode's solution with 5 mM KCl. Muscle nerves (femorotibialis external/medial, adductor) were drawn into suction electrodes for stimulation and recording. All recordings were performed at 27°C. The dissection and recordings were performed in the absence of drugs unless otherwise stated.
Pharmacological Blockade of Synaptic Transmission.
At stage 33–34 [E8 (49)], a window in the shell was opened to allow monitoring of chicken embryo limb movements and drug application. An aqueous solution of 100 μM bicuculline methiodide (Tocris Cookson), 10 μM gabazine (Tocris Cookson), 7 μM strychnine hydrochloride (Sigma–Aldrich), 20 μM CNQX (Tocris Cookson), 100 μM APV (Sigma–Aldrich), or some combination of these antagonists was injected onto the chorioallantoic membrane of the chicken embryo.
Electrophysiology.
Whole-cell voltage clamp recordings of mPSCs were obtained from antidromically identified femorotibialis or adductor motoneurons as described in ref. 7. Muscle nerve recordings were obtained from suction electrodes connected to high-gain differential amplifiers (A-M Systems). Whole-cell currents were acquired by using an AxoClamp 2B amplifier controlled by pClamp 10.1 software (Axon Instruments) running on an Apple computer. Only cells with a stable resting membrane potential were used for analysis (MiniAnalysis; Synaptosoft).
Synaptic strength was assessed by measuring the amplitude and the frequency of spontaneous postsynaptic currents (sPSCs). Previous studies using TTX have demonstrated that the majority of sPSCs recorded are action potential-independent mPSCs (7, 44). Therefore, experiments were performed without TTX (except where mentioned) and are referred to as mPSCs. Previous reports have suggested that motoneurons in the E10 chicken embryo primarily receive GABAergic and glutamatergic mPSCs (7, 44). To establish which populations of neurotransmitters contributed to the mPSCs recorded, GABAergic and glutamatergic antagonists were acutely bath-applied. We find that mPSCs in femorotibialis and adductor motoneurons at E10 are composed of only two populations: AMPA mPSCs and GABA mPSCs (see Table 1). Previous studies suggested that AMPA and GABA mPSCs could be isolated kinetically (7, 50, 51). We found that in the chicken embryo slow-decay mPSCs are largely GABA-mediated, whereas fast-decay mPSCs are largely AMPA-mediated. We found that only 6.6% of mPSCs with decay constants >23 ms (presumptive GABAergic) are AMPAergic (based on pharmacologically isolated mPSCs in those cells, n = 4). Only 7.5% of mPSCs with decay constants <21 ms (presumptive AMPAergic) are GABAergic (n = 3). Therefore, we kinetically separated contributions from GABAergic (τ ≥23 ms) and AMPAergic mPSCs (τ ≤21 ms) without pharmacological intervention. Currents with decay times between 21 ms and 23 ms (21 < τ < 23) were not included in further analysis.
Statistics.
Data from averages are expressed as mean ± SEM. Statistical analyses of mPSC amplitudes were performed by using ANOVA with post hoc Tukey's test (α = 0.05). For cumulative distributions, best-fit analysis was performed by using a Kolmogorov–Smirnov test (α = 0.05).
Acknowledgments.
We thank Drs. Mark Rich, Carlos Gonzalez-Islas, and Michael O'Donovan for critical comments on the manuscript. This work was supported by National Institute of Neurological Disorders and Stroke/National Institutes of Health Grant NS-046510 and National Science Foundation Grant 0616097 (to P.W.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 2.Feller MB. Spontaneous correlated activity in developing neural circuits. Neuron. 1999;22:653–656. doi: 10.1016/s0896-6273(00)80724-2. [DOI] [PubMed] [Google Scholar]
- 3.O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 4.O'Donovan MJ, Chub N, Wenner P. Mechanisms of spontaneous activity in developing spinal networks. J Neurobiol. 1998;37:131–145. doi: 10.1002/(sici)1097-4695(199810)37:1<131::aid-neu10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Casavant RH, Colbert CM, Dryer SE. A-current expression is regulated by activity but not by target tissues in developing lumbar motoneurons of the chick embryo. J Neurophysiol. 2004;92:2644–2651. doi: 10.1152/jn.00307.2004. [DOI] [PubMed] [Google Scholar]
- 6.Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Islas C, Wenner P. Spontaneous network activity in the embryonic spinal cord regulates AMPAergic and GABAergic synaptic strength. Neuron. 2006;49:563–575. doi: 10.1016/j.neuron.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci. 2007;30:119–125. doi: 10.1016/j.tins.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Turrigiano G. Homeostatic signaling: The positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien RJ, et al. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 12.Turrigiano GG, et al. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 13.Davis GW. Homeostatic control of neural activity: From phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, et al. A model neuron with activity-dependent conductances regulated by multiple calcium sensors. J Neurosci. 1998;18:2309–2320. doi: 10.1523/JNEUROSCI.18-07-02309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marder E, Prinz AA. Modeling stability in neuron and network function: The role of activity in homeostasis. Bioessays. 2002;24:1145–1154. doi: 10.1002/bies.10185. [DOI] [PubMed] [Google Scholar]
- 16.Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci. 2007;27:8268–8277. doi: 10.1523/JNEUROSCI.1738-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 18.Hartman KN, et al. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- 19.Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 20.Chub N, Mentis GZ, O'Donovan MJ. Chloride-sensitive MEQ fluorescence in chick embryo motoneurons following manipulations of chloride and during spontaneous network activity. J Neurophysiol. 2006;95:323–330. doi: 10.1152/jn.00162.2005. [DOI] [PubMed] [Google Scholar]
- 21.Marchetti C, et al. Modeling spontaneous activity in the developing spinal cord using activity-dependent variations of intracellular chloride. J Neurosci. 2005;25:3601–3612. doi: 10.1523/JNEUROSCI.4290-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chub N, O'Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J Neurosci. 1998;18:294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marder E, Tobin AE, Grashow R. How tightly tuned are network parameters? Insight from computational and experimental studies in small rhythmic motor networks. Prog Brain Res. 2007;165:193–200. doi: 10.1016/S0079-6123(06)65012-7. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Ari Y, et al. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 25.Pflieger JF, Clarac F, Vinay L. Picrotoxin and bicuculline have different effects on lumbar spinal networks and motoneurons in the neonatal rat. Brain Res. 2002;935:81–86. doi: 10.1016/s0006-8993(02)02469-1. [DOI] [PubMed] [Google Scholar]
- 26.Bai D, et al. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 27.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 28.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 29.Thiagarajan TC, Piedras-Renteria ES, Tsien RW. α- and β-CaMKII inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 30.Landmesser LT, O'Donovan MJ. Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol (London) 1984;347:189–204. doi: 10.1113/jphysiol.1984.sp015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Donovan MJ. Motor activity in the isolated spinal cord of the chick embryo: Synaptic drive and firing pattern of single motoneurons. J Neurosci. 1989;9:943–958. doi: 10.1523/JNEUROSCI.09-03-00943.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provine RR. Ontogeny of bioelectric activity in the spinal cord of the chick embryo and its behavioral implications. Brain Res. 1972;41:365–378. doi: 10.1016/0006-8993(72)90508-2. [DOI] [PubMed] [Google Scholar]
- 33.Provine RR, Aloe L, Seshan KR. Spontaneous bioelectric activity in long term cultures of the embryonic insect central nervous system. Brain Res. 1973;56:364–370. doi: 10.1016/0006-8993(73)90354-5. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Ari Y, et al. Interneurons set the tune of developing networks. Trends Neurosci. 2004;27:422–427. doi: 10.1016/j.tins.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Trombley PQ, van den Pol AN. GABA receptors precede glutamate receptors in hypothalamic development; differential regulation by astrocytes. J Neurophysiol. 1995;74:1473–1484. doi: 10.1152/jn.1995.74.4.1473. [DOI] [PubMed] [Google Scholar]
- 36.Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30:382–389. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Kandler K, Gillespie DC. Developmental refinement of inhibitory sound-localization circuits. Trends Neurosci. 2005;28:290–296. doi: 10.1016/j.tins.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 39.Lissin DV, et al. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci USA. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turrigiano GG, Nelson SB. Thinking globally, acting locally: AMPA receptor turnover and synaptic strength. Neuron. 1998;21:933–935. doi: 10.1016/s0896-6273(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 41.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 42.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 43.Leslie KR, Nelson SB, Turrigiano GG. Postsynaptic depolarization scales quantal amplitude in cortical pyramidal neurons. J Neurosci. 2001;21:RC170. doi: 10.1523/JNEUROSCI.21-19-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chub N, O'Donovan MJ. Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo. J Neurophysiol. 2001;85:2166–2176. doi: 10.1152/jn.2001.85.5.2166. [DOI] [PubMed] [Google Scholar]
- 45.Frank CA, et al. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- 47.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 48.Wenner P, O'Donovan MJ. Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J Neurophysiol. 2001;86:1481–1498. doi: 10.1152/jn.2001.86.3.1481. [DOI] [PubMed] [Google Scholar]
- 49.Hamburger V, Hamilton HC. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 50.Galante M, Nistri A, Ballerini L. Opposite changes in synaptic activity of organotypic rat spinal cord cultures after chronic block of AMPA/kainate or glycine and GABAA receptors. J Physiol (London) 2000;523:639–651. doi: 10.1111/j.1469-7793.2000.t01-1-00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiner JA, et al. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]