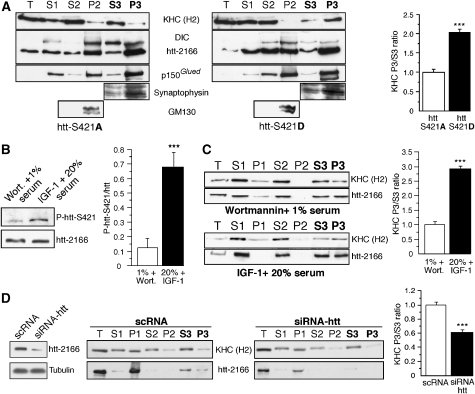
Figure 3.
Phosphorylation of htt leads to a recruitment of kinesin-1 to vesicles. (A) Subcellular fractionation of mouse neuronal cell extracts by successive centrifugation steps is analysed by immunoblotting for the presence of kinesin-1 (KHC), dynein (DIC), htt, p150Glued, GM130 (Golgi marker) and synaptophysin (small vesicles marker). Quantitative assessment of the optical density of KHC is expressed as P3/S3 ratio and shows a recruitment of KHC from cytosol to vesicles in cells expressing htt-S421D. (B) Phosphorylation of endogenous htt at S421 is increased by IGF-1 treatment. Total extracts from mouse neuronal cells treated with wortmannin (wort)/1% serum or IGF-1/20% serum are immunoprobed with the anti-phospho-htt-S421–714 and anti-htt antibodies. Quantification of phosphorylated htt (P-htt) is expressed as a ratio of P-htt/total htt optical densities. (C) Phosphorylation of endogenous htt leads to kinesin-1 recruitment to vesicles. Extracts treated as in (B) are subjected to subcellular fractionation, analysed and quantified as in (A). (D) Loss of htt reduces kinesin-1 recruitment to vesicles. Mouse neuronal cells treated with siRNA targeting htt or control scramble RNA (scRNA) were subjected to subcellular fractionation, analysed and quantified as in (A). (***P<0.001; see Supplementary data for detailed statistical analyses and number of measures).