Figure 5.
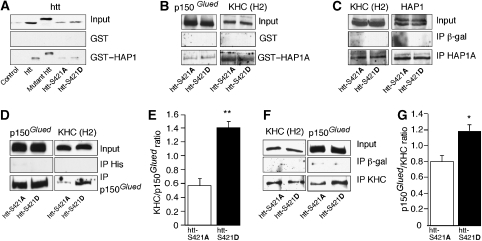
Phosphorylation of huntingtin at S421 modifies the nature of the molecular motor complexes. (A) GST–HAP1 pull-down experiments of protein extracts from HEK cells transfected with htt, mutant htt-polyQ, htt-S421A or htt-S421D reveal that the binding of HAP1 with the htt 480 amino-acid fragment is not modulated by phosphorylation. (B) GST–HAP1A pull-down experiments from HEK cells transfected with htt-S421A or htt-S421D show that the interactions between HAP1A and p150Glued and between HAP1A and KHC are not modified by phosphorylation. (C) The HAP1A–KHC interaction is unchanged whether htt S421 is phosphorylated or not. Extracts of htt-S421A- and htt-S421D-transfected mouse neuronal cells are immunoprecipitated using anti-β-galactosidase (control) or anti-HAP1A antibodies and immunoprobed with anti-KHC (H2) and anti-HAP1 (EM78) antibodies. (D–G) The p150Glued–KHC interaction is significantly increased when S421 is phosphorylated. (D) Mouse neuronal cell extracts are immunoprecipitated using anti-His (control) or anti-p150Glued antibodies and immunoprobed with anti-p150Glued and anti-KHC antibodies. (F) Mouse neuronal cell extracts are immunoprecipitated using anti-β-galactosidase (control) or anti-KHC antibodies and immunoprobed as in (D). Quantitative assessments of the optical densities were performed and expressed as KHC/p150Glued (E) or p150Glued/KHC ratios (G) (*P<0.05, **P<0.01; see Supplementary data for detailed statistical analyses and number of measures).