Figure 4.
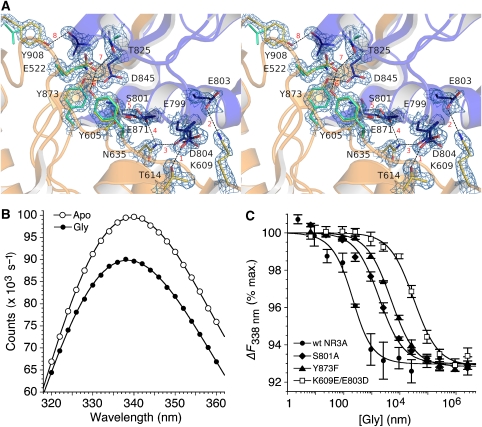
Interdomain interactions stabilize the closed-cleft conformation of NR3A. (A) Stereo view of the NR3A glycine complex with domains 1 and 2 coloured gold and blue, respectively. The electron density map (2mFo−DFc contoured at 2σ) reveals interdomain hydrogen bonds and salt bridges at eight sites, which are individually numbered in red. Several of these contacts are absent in NR1, owing to replacement of Tyr side chains by Phe and Val; these are coloured pale green for the NR1 glycine crystal structure (1PB7). (B) Quenching of intrinsic tryptophan fluorescence for the NR3A Glu871S mutant following the addition of 300 μM glycine (≈100 × Kd). (C) Fluorescence quenching curves measured at 338 nm, with excitation at 282 nm, for wild-type NR3A and the NR3A S801A, Y873F, and K609E/E803D mutants; data points show mean and s.e.m. of three experiments.