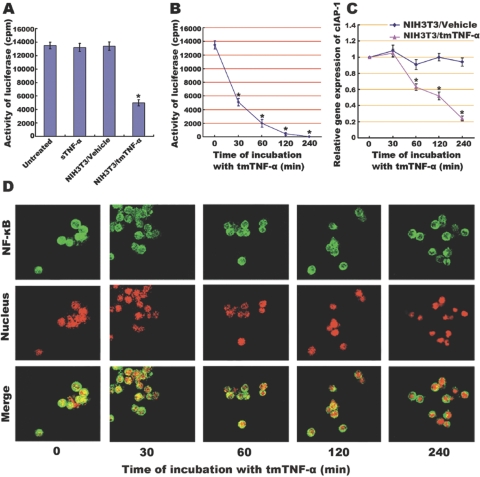
Fig. 2.
Inhibitory effect of tmTNF-α on NF-κB activation and NF-κB-mediated transcription of cIAP-1 in Raji cells, which (A) when transiently cotransfected with a 3× NF-κB–luciferase reporter construct and a β-galactosidase expression vector, were stimulated for 30 min with sTNF-α (100 U/ml) or with fixed NIH3T3 cells transfected with tmTNF-α (at an E:T ratio of 10:1). Cells treated with nontransfection and left untreated served as controls. Luciferase activity was measured and normalized against the β-galactosidase activity. Data represent the mean of three independent experiments. Time course of inhibitory effect of tmTNF-α on NF-κB activation and cIAP transcription was performed by incubation of the Raji cells transfected by 3× NF-κB–luciferase reporter with fixed NIH3T3 cells (at an E:T ratio of 10:1) transfected with tmTNF-α or empty plasmid for the indicated time-points. Luciferase activity was measured, and data represent the mean of three independent experiments (B). The transcription level of cIAP-1 was analyzed by real-time RT-PCR. The results shown are representative of three independent experiments (C). The translocation of NF-κB in Raji cells was detected by confocal microscopy (D). An anti-NF-κB/p65 polyclonal antibody, a FITC-labeled, anti-IgG antibody, and PI nuclear counterstain were used to stain cells. NF-κB (green stain), translocated from the cytoplasm to the nucleus (red stain), showed as yellow fluorescence in the merged images, which were representative of three slides scanned; *, P < 0.01.