Table 1.
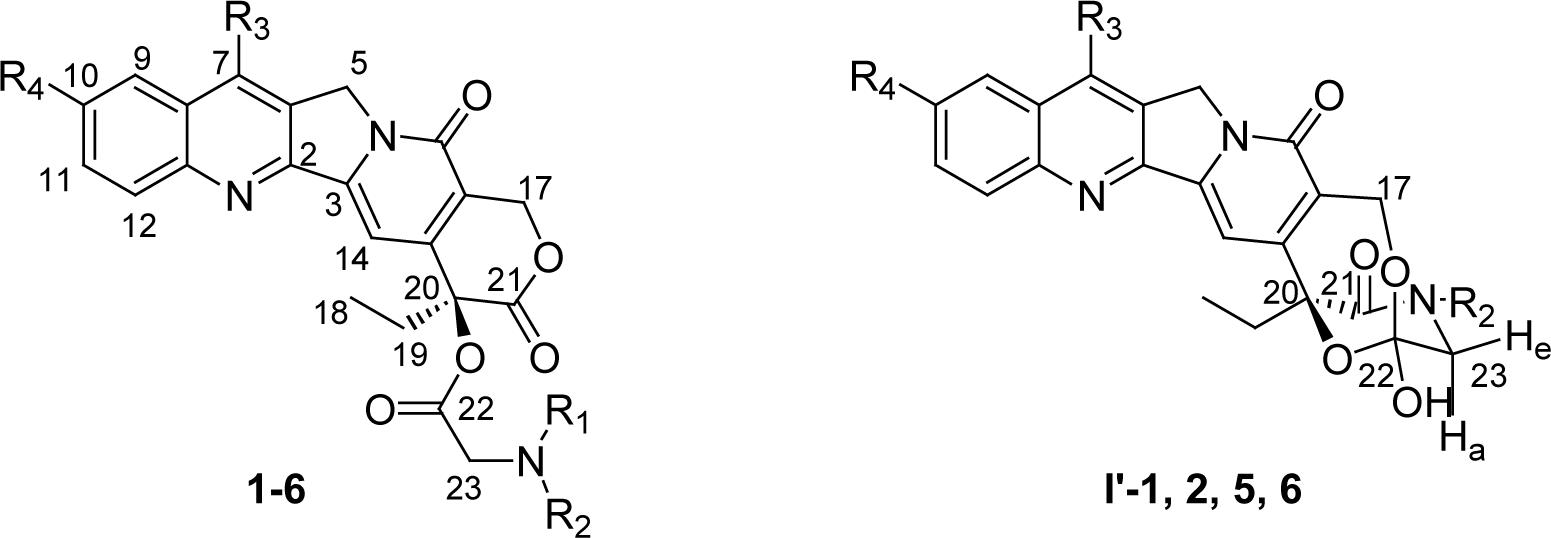
Selected 13C and 1H NMR chemical shifts (ppm) for various α-amino acid esters of CPT and DB-67 and their corresponding hemiorthoesters (I′).
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
|
|
|
|
NMR assignment |
|
|
||
| Cmpd | R1 | R2 | R3 | R4 | C-17 | C-20 | C-22 | C-23 | H-17 | H-23 | OHa |
| 1 | H | H | H | H | 66.3 | 77.4 | 167.0 | 50.2 | 5.56 (s) | 4.08−4.39 (AB) | |
| I′-1 | - | H | H | H | 57.4 | 81.9 | 107.4 | 47.8 | 4.85−5.05 (AB) | 3.33−3.49 (ABX) | 7.93 |
| 2 | H | CH3 | H | H | 66.4 | 77.7 | 166.8 | 47.9 | 5.56 (s) | 4.30−4.47 (AB) | |
| I′-2 | - | CH3 | H | H | 57.4 | 82.0 | 107.1 | 54.8 | 4.83−5.06 (AB) | 3.41−3.72 (AB) | 8.06 |
| 3 | CH3 | CH3 | H | H | 66.4 | 78.0 | - | 50.3 | 5.57 (s) | 4.53−4.63 (AB) | |
| 4 | H | COCH3 | H | H | 67.0 | 77.4 | 167.2 | 41.5 | 5.40−5.70 (AB) | 4.15−4.45 (ABX) | |
| 5 | H | H | TBS | OH | 66.3 | 77.5 | 166.8 | 52.2 | 5.52 (s) | 4.10−4.31 (AB) | |
| I′-5 | - | H | TBS | OH | - | - | - | - | 4.73−5.12 (AB) | 3.28−3.48 (ABX) | 7.88 |
| 6 | H | CH3 | TBS | OH | 67.8 | 80.0 | - | 54.3 | 5.48−5.63 (AB) | 4.30−4.38 (AB) | |
| I′-6 | - | CH3 | TBS | OH | 57.3 | 82.0 | 107.0 | 52.0 | 4.73−5.11 (AB) | 3.41−3.69 (AB) | 7.96 |
22-Ortho hydroxyl group of I′
