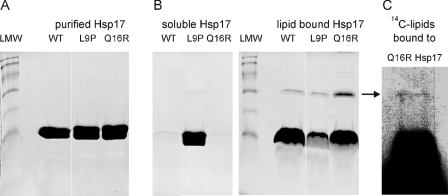
FIGURE 2.
Binding of Hsp17 proteins to liposomes made of total polar lipids isolated from heat-treated cells. A, purity of recombinant proteins was assayed by SDS-PAGE and revealed by Coomassie blue staining. Low molecular weight markers (LMW) display protein bands referring to molecular masses of 94, 67, 43, 30, 20, and 14 kDa, respectively. B, purified proteins were incubated with liposomes for 1 h and then fractionated into lipid bound (pellet) and soluble (supernatant) fractions. The samples were solubilized under reducing conditions and analyzed by SDS-PAGE and Coomassie blue staining. C, the binding assay was repeated with vesicles made of 14C-labeled lipids. The arrow indicates the band corresponding to the Hsp17 associated with 14C-labeled lipids. Lipid content of the pelleted Q16R Hsp17 fraction solubilized under reducing conditions was revealed by fluorography.