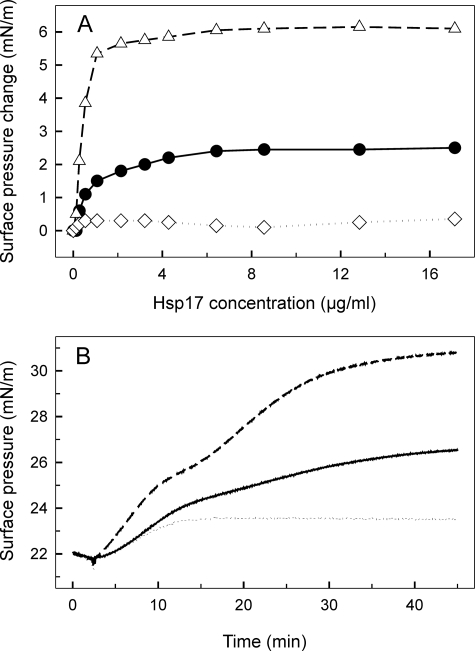
FIGURE 3.
Interaction of WT and mutant Hsp17 proteins with monomolecular lipid layers of total polar lipids (A) and SQDG (B) isolated from heat-treated cells. A, increasing concentrations of Hsp17 proteins (WT, Q16R, and L9P as circles, triangles, and diamonds, respectively) were injected underneath lipid layers, and equilibrium surface pressures were recorded. B, 4.2 μg/ml of each sHsp (WT, Q16R, and L9P as solid, dashed, and dotted lines, respectively) was injected into the buffer beneath the lipid layer, and development of protein-lipid binding was monitored by following the surface pressure increase.