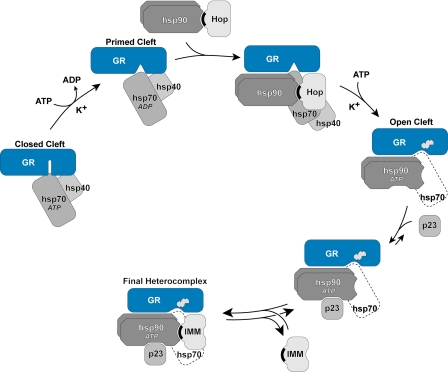
FIGURE 1.
Mechanism of cleft opening and GR·Hsp90·immunophilin heterocomplex assembly. The ATP-dependent conformation of Hsp70 binds initially to the GR, and in an ATP-, K+-, and Hsp40-dependent step, a GR·Hsp70 complex is formed that is primed to interact with Hsp90. After Hsp90 binding, there is a second ATP- and K+-dependent step that is rate-limiting and leads to opening of the steroid binding cleft, enabling access of the steroid (indicated by the steroid structure). During GR·Hsp90 heterocomplex assembly in cells and cell lysates, Hop and some of Hsp70 dissociate during or at the end of the cleft opening step. The GR-bound Hsp90 is now in its ATP-dependent conformation and can be bound by p23, which stabilizes the chaperone in that conformation, preventing disassembly of the GR·Hsp90 heterocomplex. When Hop dissociates, TPR domain proteins, such as immunophilins (IMM), can bind reversibly to the TPR acceptor site on GR-bound Hsp90. TPR domains are indicated by black crescents.