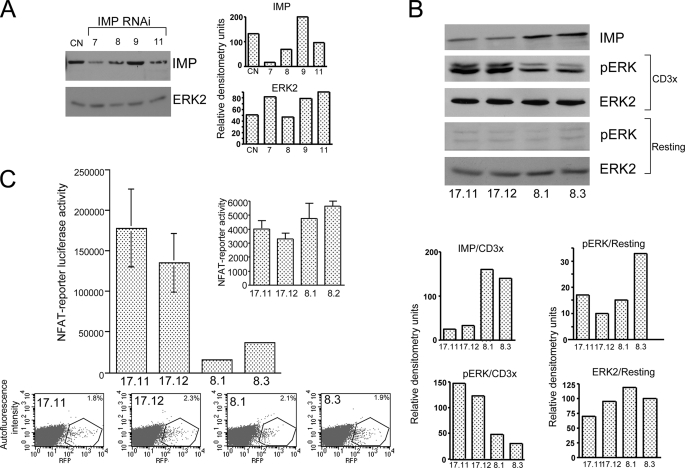
FIGURE 1.
Imp represses the transcriptional response in human T cells. A, Western blot analysis of Jurkat T cells expressing individual RNAi designed against human Imp. Jurkat T cells were transiently electroporated with individual RNAi subcloned into the pSilcencer™ 3.1-H1 hygro and pMSCV, which expresses GFP. After 48 h, the cells were sorted for GFP expression and then treated with the 1% Nonidet P-40 lysis buffer. The cell extracts were analyzed on Western blots using anti-Imp and anti-Erk2 antibodies (see “Experimental Procedures”). B, Western blot analysis of the Imp protein level and activated ERK in individual Jurkat T clones expressing Imp-specific RNAi (number 7) or control RNAi. After a resting period in RPMI1640 medium supplemented with 0.1% fetal calf serum, the cells were left unstimulated (resting) or stimulated with anti-CD3 mAb (CD3x) (1.0 μg/ml) followed by cross-linking with 100 μg/ml goat anti-rat secondary antibody (5 min at 37 °C). Next, equal numbers of cells were lysed and the materials collected were run on the 9% acrylamide gel and analyzed by Western blotting, as indicated. C, transactivation of the NFAT reporter in Jurkat T cells expressing reduced levels of Imp. Jurkat T cell clones stably expressing either Imp-specific RNAi number 7 (clones 17.11 and 17.12) or CD8-specific RNAi (8.1 and 8.3 controls) were transfected with NFAT-luciferase reporter (top) and red fluorescent protein (RFP) to measure transfection efficiency by FACS (x axis, expression level of red fluorescent protein; y axis, autofluorescence intensity, bottom). After 48 h the cells were stimulated for an additional 18 h with the plate-bound anti-CD3 mAb (1 μg/ml) or left unstimulated (inset), harvested, and analyzed using the dual-luciferase reporter assay system (36). One representative of three experiments is shown (the data are expressed as mean ± S.D. of triplicate cultures (S.D. values for 17.11, 17.12, 8.1, and 8.2 cells are 48,386, 36,608, 1,785, and 2,024, respectively).