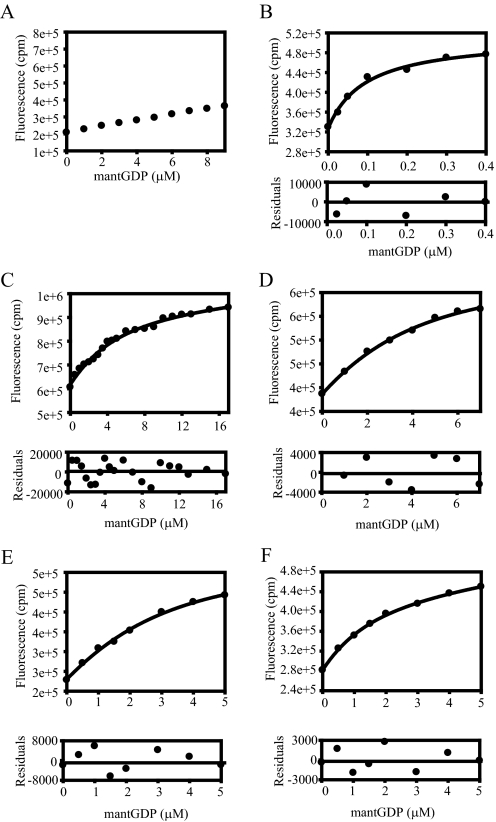
FIGURE 1.
GEF-independent mutants of eEF1A reduce GDP binding. Equilibrium dissociation constants (Kd) for the mutant forms of eEF1A and mant-GDP were measured. Aliquots of mant-GDP were added to binding buffer without (A) or with 1 μm wild type (B) or the mutant forms (C–E) of eEF1A. The fluorescence was measured by FRET via excitation at 280 nm and emission of 440 nm for mant moiety. Fluorescence intensity (cpm) versus the concentration of mant-GDP (μm) was plotted, and data (•) were fit to a hyperbolic curve to obtain the Kd value. The Kd values are measured as 3.54 (T22S) (C), 2.66 (R164K) (D), 1.80 (A112T) (E), and 1.18 μm (A117V) (F). Residuals for the fits are shown in the lower panels to detect the experimental error for the fitted data sets.