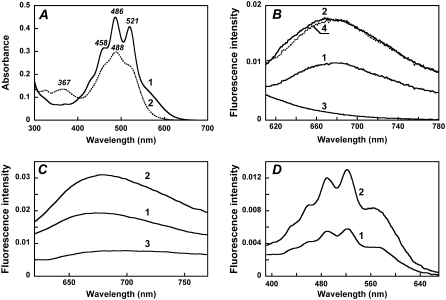
FIGURE 2.
Absorption, fluorescence, and excitation spectra of xanthorhodopsin. Effect of retinal removal with hydroxylamine. (A) Absorption spectrum of cell membranes of Salinibacter ruber containing xanthorhodopsin (at pH 5.5) before (spectrum 1) and after (spectrum 2) hydrolysis of the retinal Schiff base with hydroxylamine. The treatment converts the covalently bound retinal to the retinal oxime, whose absorption maximum is shifted from 563 nm to 367 nm, and the sharp carotenoid vibronic bands broaden. (B) Fluorescence emission of xanthorhodopsin, pH 5.5, excitation at 560 nm (spectrum 1) and 520 nm (spectrum 2). Spectrum 3, after hydroxylamine treatment and removal of the retinal oxime, excitation at 560 nm. Spectrum 4, spectrum 1 normalized to the amplitude for 520 nm excitation, by multiplying by 1.7. (C) Comparison of the xanthorhodopsin fluorescence bands at pH 8 (spectrum 1) and pH 5.5 (spectrum 2) with that of bacteriorhodopsin, pH 6 (spectrum 3). Excitation: 560 nm, 8 nm bandwidth. Absorbances of the samples at 560 nm were 0.29, 0.32, and 0.33, respectively. A small contribution from the Raman scattering of water was subtracted. (D) Fluorescence excitation spectra for the emission of xanthorhodopsin, sampled at 720 nm, at pH 8 (spectrum 1) and pH 5.5 (spectrum 2). Excitation beam bandwidth 4 nm.