Abstract
Leprosy bacilli harvested from freshly biopsied tissue from cases of lepromatous, borderline and histoid leprosy were, in conjunction with Mycobacterium lepraemurium and representative mycobacteria, examined cytochemically with and without their pyridine-extractable acid-fastness. Unlike the mycobacteria, unextracted leprosy bacilli failed to give a positive response to the periodic acid Schiff test or to take up Sudan black B, toluidine blue O, alkaline methylene blue or safranin O. Once their acid-fastness was removed with pyridine, leprosy bacilli were stained by all of the foregoing dyes except Sudan black B, under this condition they remained gram positive. While permanent loss of acid-fastness from leprosy bacilli always resulted in a loss of acid hematein-fixing material (Smith-Dietrich-Baker tests), the reverse was not true. Mild aqueous saponification, bromination, or sequential treatment with lipase and phospholipase D resulted in a loss of acid hematein-positivity but not acid-fastness. After pyridine extraction, bromination, or aqueous saponification, true mycobacteria lost neither their acid hematein-positivity nor their acid-fastness. The acid hematein-positive material and the acid-fastness of both leprosy bacilli and mycobacteria were lost after treatment with alkaline ethanol. These cytochemical findings are discussed in the light of what is known of the ultrastructure of leprosy bacilli and mycobacteria, and of the occurrence of a dl-3, 4-dihydroxyphenylalanine oxidase in leprosy bacilli but not in mycobacteria. An effort is made to explain the rather unique cytochemical properties of leprosy bacilli. Since pyridine-extractable acid-fastness (and acid hematein-positivity) serve to distinguish human leprosy bacilli from M. lepraemurium, one or the other, or both, are suggested as bases for differentiating these two organisms in animal experiments designed to show the in vivo propagation of human leprosy bacilli.
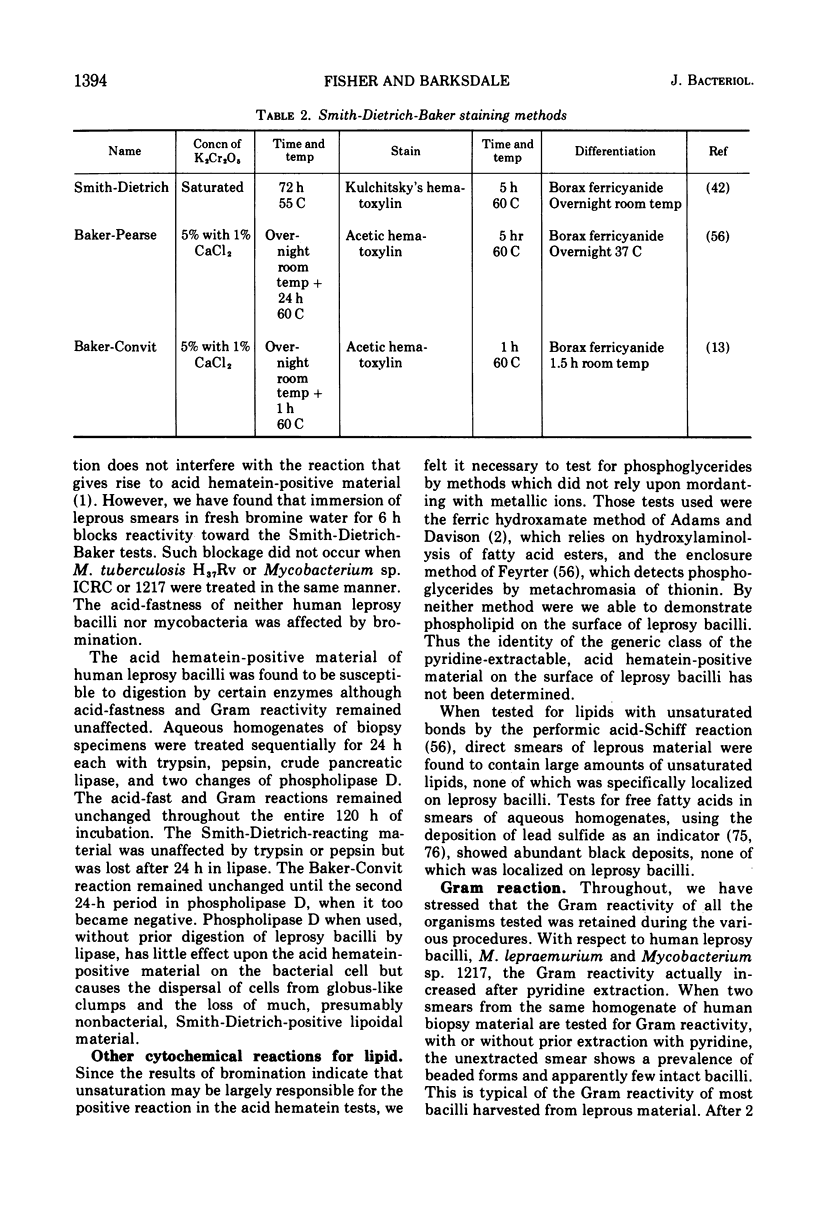
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS C. W. M., ABDULLA Y. H., BAYLISS O. B., WELLER R. O. THE REACTION OF BAKER'S CHROMATE REAGENT WITH LIPIDS. J Histochem Cytochem. 1965 May-Jun;13:410–411. doi: 10.1177/13.5.410. [DOI] [PubMed] [Google Scholar]
- ADAMS C. W., DAVISON A. N. The histochemical identification of myelin phosphoglycerides by their ferric hydroxamates. J Neurochem. 1959 Feb;3(4):347–351. doi: 10.1111/j.1471-4159.1959.tb12641.x. [DOI] [PubMed] [Google Scholar]
- BARDSDALE W. L., PAPPENHEIMER A. M., Jr Phage-host relationships in nontoxigenic and toxigenic diphtheria bacilli. J Bacteriol. 1954 Feb;67(2):220–232. doi: 10.1128/jb.67.2.220-232.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMANN R. Essais de coloration du bacille de Stefansky et du bacille de Hansen par le noir Soudan B.N. 1953 May 26-Jun 2Bull Acad Natl Med. 137(19-20):295–298. [PubMed] [Google Scholar]
- BINFORD C. H. Studies on a Mycobacterium obtained from the golden hamster (Cricetus auratus) after inoculation with lepromatous tissue. Lab Invest. 1962 Nov;11:942–955. [PubMed] [Google Scholar]
- Beaman B. L., Kim K. S., Salton M. R., Barksdale L. Amino acids of the cell wall of Nocardia rubra. J Bacteriol. 1971 Nov;108(2):941–943. doi: 10.1128/jb.108.2.941-943.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Barksdale L. Phenoloxidase activity in organisms isolated from lepromatous and tuberculoid leprosy. J Bacteriol. 1970 Dec;104(3):1406–1408. doi: 10.1128/jb.104.3.1406-1408.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon K. L. Disparity in Appearance of True Hansen's Bacilli and Cultured "Leprosy Bacilli" When Stained for Fat. J Bacteriol. 1946 Dec;52(6):679–680. [PMC free article] [PubMed] [Google Scholar]
- CASSELMAN W. G. B. The in vitro preparation and histochemical properties of substances resembling ceroid. J Exp Med. 1951 Dec 1;94(6):549–562. doi: 10.1084/jem.94.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTERJEE K. R. Experimental transmission of human leprosy infection to a selected, laboratory-bred hybrid black mouse. Int J Lepr. 1958 Jul-Sep;26(3):195–204. [PubMed] [Google Scholar]
- CHAUSSINAND R., VIETTE M. Etude de la coloration des bacilles acido-alcoolo-résistants par le noir soudan. Ann Inst Pasteur (Paris) 1955 Sep;89(3):280–289. [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. Studies on the cell-wall composition and taxonomy of Actinomycetales and related groups. J Gen Microbiol. 1958 Feb;18(1):173–189. doi: 10.1099/00221287-18-1-173. [DOI] [PubMed] [Google Scholar]
- Campo-Aasen I., Convit J. Identification of the noncultivable pathogenic mycobacteria M. leprae and M. lepraemurium. Int J Lepr Other Mycobact Dis. 1968 Apr-Jun;36(2):166–170. [PubMed] [Google Scholar]
- Edwards R. P. Electron-microscope illustrations of division in Mycobacterium leprae. J Med Microbiol. 1970 Aug;3(3):493–499. doi: 10.1099/00222615-3-3-493. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J Biol Chem. 1951 Aug;191(2):807–817. [PubMed] [Google Scholar]
- Fisher C. A., Barksdale L. Elimination of the acid fastness but not the gram positivity of leprosy bacilli after extraction with pyridine. J Bacteriol. 1971 May;106(2):707–708. doi: 10.1128/jb.106.2.707-708.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J., Kim K. S., Krauss M. R., Beaman L., Barksdale L. Ultrastructural changes in bacteria isolated from cases of leprosy. J Bacteriol. 1969 Nov;100(2):1062–1075. doi: 10.1128/jb.100.2.1062-1075.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., White R. G. Surface peptido-glycolipid filaments on Mycobacterium leprae. Clin Exp Immunol. 1971 Oct;9(4):539–547. [PMC free article] [PubMed] [Google Scholar]
- HANKS J. H. Significance of capsular components of Mycobacterium leprae and other mycobacteria. Int J Lepr. 1961 Jan-Mar;29:74–83. [PubMed] [Google Scholar]
- Imaeda T., Convit J. ELECTRON MICROSCOPE STUDY OF MYCOBACTERIUM LEPRAE AND ITS ENVIRONMENT IN A VESICULAR LEPROUS LESION. J Bacteriol. 1962 Jan;83(1):43–52. doi: 10.1128/jb.83.1.43-52.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda T. Electron microscopy. Approach to leprosy research. Int J Lepr. 1965 Jul-Sep;33(3 Suppl):669–688. [PubMed] [Google Scholar]
- Imaeda T., Kanetsuna F., Rieber M., Galindo B., Cesari I. M. Ultrastructural characteristics of mycobacterial growth. J Med Microbiol. 1969 Aug;2(3):181–186. doi: 10.1099/00222615-2-3-181. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Barksdale L. Crystalline inclusions of bacterium 22M. J Bacteriol. 1969 Jun;98(3):1390–1394. doi: 10.1128/jb.98.3.1390-1394.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingmüller G., Orfanos C. Der elektronenmikroskopische Aufbau des Mycobacterium Leprae. Arch Klin Exp Dermatol. 1966 Apr 22;224(4):373–384. [PubMed] [Google Scholar]
- McRae D. H., Shepard C. C. Relationship Between the Staining Quality of Mycobacterium leprae and Infectivity for Mice. Infect Immun. 1971 Jan;3(1):116–120. doi: 10.1128/iai.3.1.116-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murohashi T., Kondo E., Yoshida K. The role of lipids in acid-fastness of mycobacteria. Am Rev Respir Dis. 1969 May;99(5):794–798. doi: 10.1164/arrd.1969.99.5.794. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Tsuchiya T., Nagamatsu T., Aono Y., Ishida M. Staining conditions influencing morphological index of acid-fast bacilli. Kurume Med J. 1968;15(1):39–41. doi: 10.2739/kurumemedj.15.39. [DOI] [PubMed] [Google Scholar]
- Nyka W. Influence of oxidation and reduction on the acid-fastness of mycobacteria. Infect Immun. 1971 Oct;4(4):513–515. doi: 10.1128/iai.4.4.513-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn S. R., Verdoolaege-van Loo G. Isolation of a strain of Mycobacterium lepraemurium from normal laboratory mice. Ann Soc Belges Med Trop Parasitol Mycol. 1969;49(5):465–468. [PubMed] [Google Scholar]
- Prabhakaran K., Kirchheimer W. F., Harris E. B. Oxidation of phenolic compounds by Mycobacterium leprae and inhibition of phenolase by substrate analogues and copper chelators. J Bacteriol. 1968 Jun;95(6):2051–2053. doi: 10.1128/jb.95.6.2051-2053.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran K., Kirchheimer W. F. Use of 3,4-Dihydroxyphenylalanine Oxidation in the Identification of Mycobacterium leprae. J Bacteriol. 1966 Oct;92(4):1267–1268. doi: 10.1128/jb.92.4.1267-1268.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES R. J., VALENTINE R. C. The appearance of dead leprosy bacilli by light and electron microscopy. Int J Lepr. 1962 Jan-Mar;30:1–9. [PubMed] [Google Scholar]
- REES R. J., VALENTINE R. C., WONG P. C. Application of quantitative electron microscopy to the study of Mycobacterium lepraemurium and M. leprae. J Gen Microbiol. 1960 Apr;22:443–457. doi: 10.1099/00221287-22-2-443. [DOI] [PubMed] [Google Scholar]
- Ridley M. J., Ridley D. S. Stain techniques and the morphology of Mycobacterium leprae. Lepr Rev. 1971 Jun;42(2):88–95. [PubMed] [Google Scholar]
- Roozemond R. C. The staining and chromium binding of rat brain tissue and of lipids in model systems subjected to Baker's acid hematein technique. J Histochem Cytochem. 1971 Apr;19(4):244–251. doi: 10.1177/19.4.244. [DOI] [PubMed] [Google Scholar]
- SHEPARD C. C., MCRAE D. H. MYCOBACTERIUM LEPRAE IN MICE: MINIMAL INFECTIOUS DOSE, RELATIONSHIP BETWEEN STAINING QUALITY AND INFECTIVITY, AND EFFECT OF CORTISONE. J Bacteriol. 1965 Feb;89:365–372. doi: 10.1128/jb.89.2.365-372.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Imi M. The surface structure of M. leprae. Int J Lepr Other Mycobact Dis. 1968 Jul-Sep;36(3):303–308. [PubMed] [Google Scholar]
- Sato S., Okura E. Electron microscopic study of cell division in M. leprae and M. lepraemurium. Sci Rep Res Inst Tohoku Univ Med. 1967 Nov;14(3):122–129. [PubMed] [Google Scholar]
- TAKEYA K., HISATSUNE K., INOUE Y. Mycobacterial cell walls. II. Chemical composition of the "basal layer". J Bacteriol. 1963 Jan;85:24–30. doi: 10.1128/jb.85.1.24-30.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEYA K., HISATSUNE K. Mycobacterial cell walls. I. Methods of preparation and treatment with various chemicals. J Bacteriol. 1963 Jan;85:16–23. doi: 10.1128/jb.85.1.16-23.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEYA K., MORI R., KOIKE M., TODA T. Paired fibrous structure in mycobacteria. Biochim Biophys Acta. 1958 Oct;30(1):197–198. doi: 10.1016/0006-3002(58)90265-8. [DOI] [PubMed] [Google Scholar]
- TAKEYA K., MORI R., TOKUNAGA T., KOIKE M., HISATSUNE K. Further studies on the paired fibrous structure of mycobacterial cell wall. J Biophys Biochem Cytol. 1961 Feb;9:496–501. doi: 10.1083/jcb.9.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR C. E. Specificity of the Dharmendra antigen as tested in guinea-pigs. Int J Lepr. 1962 Apr-Jun;30:175–190. [PubMed] [Google Scholar]
- TUQAN N. A., ADAMS C. W. Histochemistry of myelin I. Proteins and lipid-protein complexes in the normal sheath. J Neurochem. 1961 Mar;6:327–333. doi: 10.1111/j.1471-4159.1961.tb13483.x. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., PISANO M. A new staining technique for polar bodies. J Bacteriol. 1950 Mar;59(3):357–360. doi: 10.1128/jb.59.3.357-360.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersema J. P., Binford C. H., Chang Y. T. Nerve involvement. Comparison of experimental infections by Mycobacterium leprae and Mycobacterium lepraemurium. Int J Lepr. 1965 Jul-Sep;33(3 Suppl):617–633. [PubMed] [Google Scholar]


