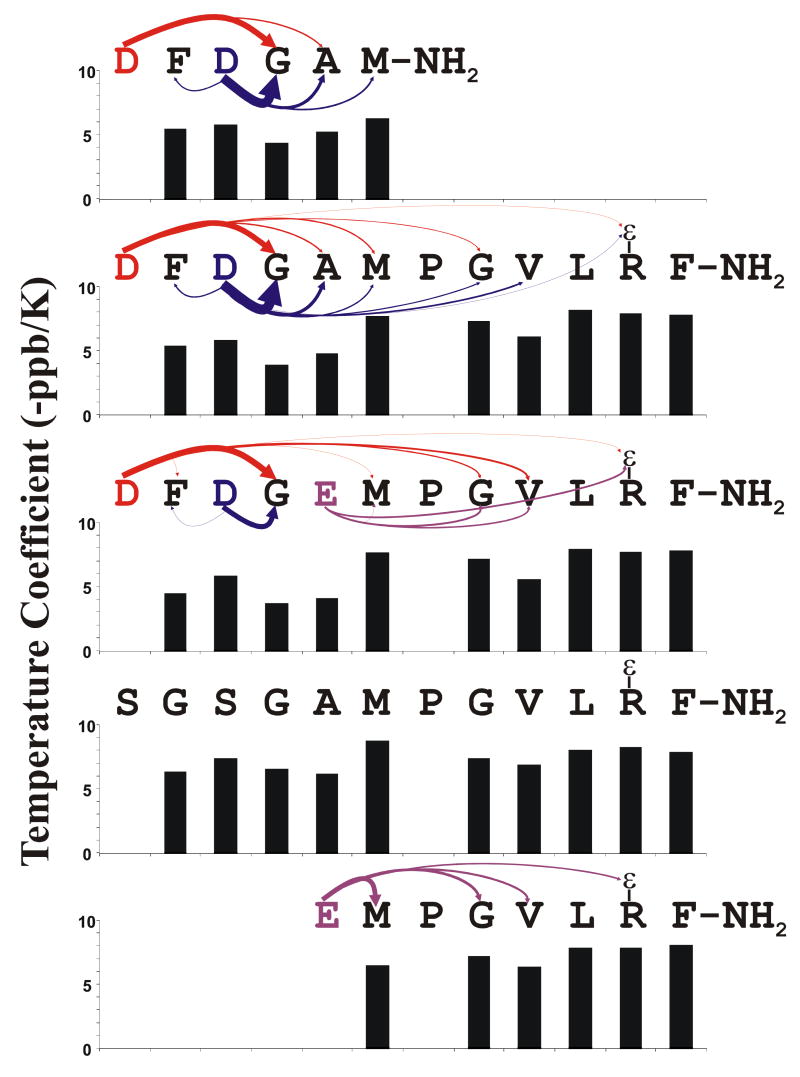
Figure 5.
Proposed H-bonding Interactions Between Backbone Amide Protons and Carboxyl Side-chains: DFDGAM-NH2 = Peptide 3, DFDGAMPGVLRF-NH2 = Peptide 2, DFDGEMPGVLRF-NH2 = Peptide 4, SGSGAMPGVLRF-NH2 = Peptide 5, EMPGVLRF-NH2 = Peptide 2. Each H-bond acceptor residue is color coded to match the arrows leading from it to its H-bond donors. The arrow widths are proportional to the relative extent to which that particular interaction affects the chemical shift of the amide proton at the point end of the arrow. The bar plots show the temperature coefficient of the backbone amide proton resonances