Figure 8.
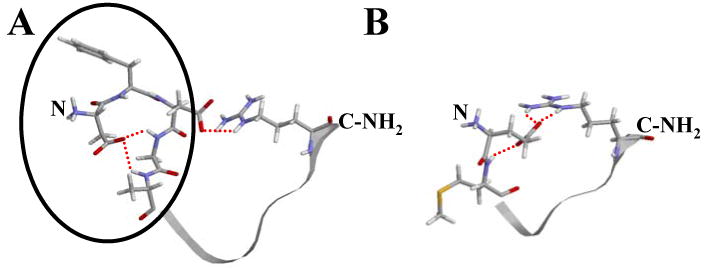
Model of interactions thought occurring within native FLP-18 peptides. This figure shows the most significant H-bonding interactions supported by NMR data. A: For DFDGAMPGVLRF-NH2, H-bonds between the D1 side-chain carboxylate and G4/A5 backbone amide protons as well as H-bonding/ionic interaction between the D3 side-chain by red dashed lines. The N-terminal loop structure implicated in inhibiting binding to NPR-1 is circled in black. B: EMPGVLRF-NH2 is shown with the most significant H-bonding and ionic interactions for which we have evidence. Notice that it has no N-terminal loop, in contrast to DFDGAMPGVLRF-NH2. Also, the same unstructured region for both peptides is shown in ribbon view. The N- and C- termini are also labeled on both peptides.
