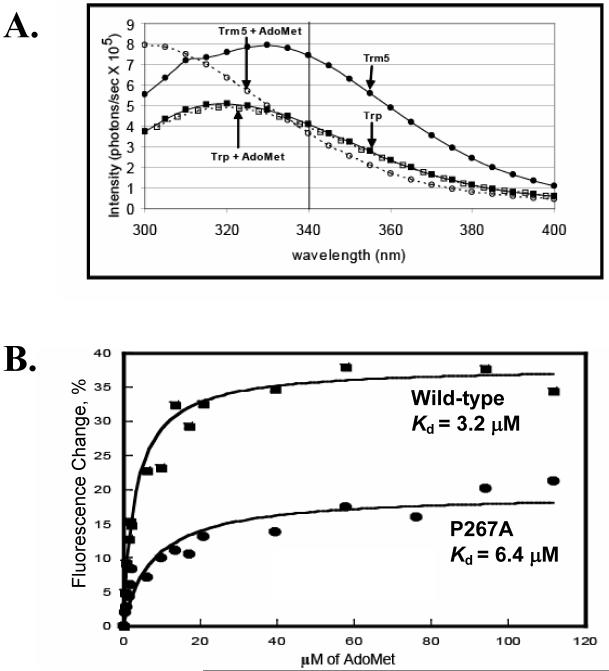
Fig.3.
(A) Fluorescence emission spectra of the wild-type Trm 5 (1.0 μM) excited at 282 nm, and the quench of the emission spectra by addition of AdoMet (100 μM) is shown. The control emission spectra of the free tryptophan solution (1.0 μM) with and without AdoMet (100 μM) were included for comparison. (B) Fitting the quench of fluorescence emission at 340 nm versus the concentration of AdoMet to a hyperbola binding equation to derive the Kd for the wild-type and P267A mutant.