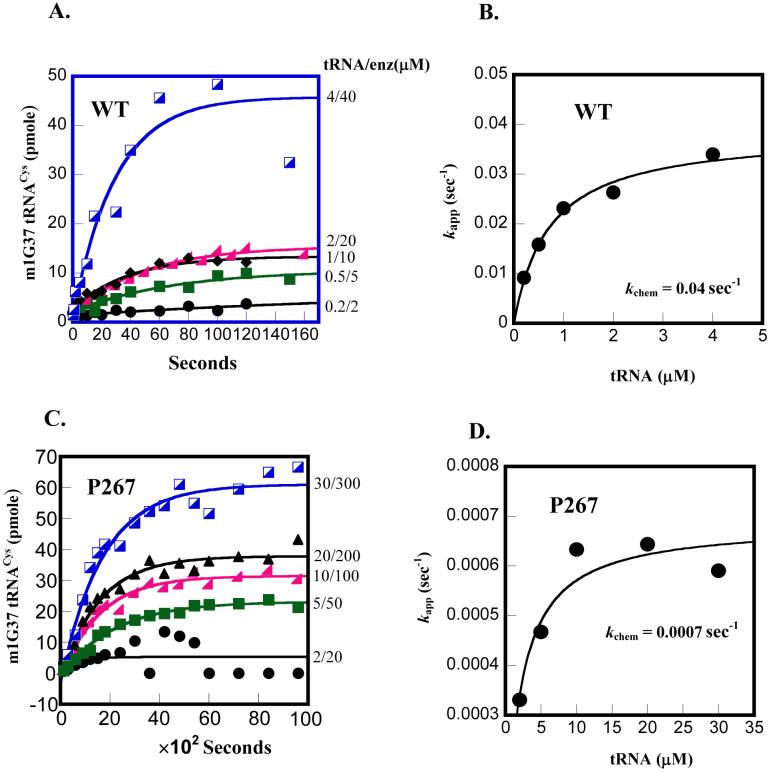
Fig.5.
Determination of kchem by single turnover kinetics. (A) Time courses of the m1G37 methyl transfer reaction performed at the indicated tRNA and enzyme concentrations for the wild-type enzyme: 0.2 μM tRNA/2 μM enzyme, 0.5 μM tRNA/5 μM enzyme, 1.0 μM tRNA/10 μM enzyme, 2 μM tRNA/20 μM enzyme, 4 μM tRNA/40 μM enzyme. (B) Replot of the data by a hyperbolic fit to derive the kchem for the wild-type. (C) Time courses of the m1G37 methyl transfer reaction performed at the indicated tRNA and enzyme concentrations for the P267A enzyme: 2 μM tRNA/20 μM enzyme, 5 μM tRNA/50 μM enzyme, 10 μM tRNA/100 μM enzyme, 20 μM tRNA/200 μM enzyme, 40 μM tRNA/400 μM enzyme. (D) Replot of the data by a hyperbolic fit to derive the kchem for the P267A mutant.