Abstract
Summary
The immune response induced by Toxoplasma gondii is characterized by Th1 immune mechanisms. We previously demonstrated that C57BL/6 mice infested with Myocoptes musculinus and infected with T. gondii by intraperitoneal route undergo accelerated mortality according to Th2 immune mechanisms induced by the acarian. To evaluate whether infection with M. musculinus influences T. gondii-induced Th1 response in a resistant mouse lineage, BALB/c, which develops latent chronic toxoplasmosis in a way similar to that observed in immunocompetent humans, this study was done. The animals were infected with T. gondii ME-49 strain 1 month after M. musculinus infestation, being the survival and the immune response monitored. The double-infected displayed higher mortality rate if compared with the mono-infected mice. In addition, infection with M. musculinus changed the T. gondii-specific immune response, converting BALB/c host to a susceptible phenotype. Spleen cells had increased the levels of IL-4 in double-infected mice. This alteration was associated with severe pneumonia, encephalitis and wasting condition. In addition, a higher tissue parasitism was observed in double-infected animals. It can be concluded that infection with these two contrasting parasites, M. musculinus and T. gondii, may convert an immunocompetent host into a susceptible one, and such a host will develop severe toxoplasmosis.
Keywords: BALB/c mice, coinfection, Myocoptes musculinus, Toxoplasma gondii
The Infestation of mice with mites is relatively common in research laboratories, where mice are kept under conventional conditions (Jungmann et al. 1996a; Matsuda et al. 1997). Mite-associated ulcerative dermatitis (MAUD) has been described in C57BL/6 mice and has been associated with Myobia musculi infestation (Dawson et al. 1986). A related disease in BALB/c mice is caused by infestation with the mite Myocoptes musculinus, and it is accompanied by a T-helper-2 (Th-2) type response (Jungmann et al. 1996b) and allergic manifestations, such as erythematous and pruritic skin lesions, widespread hair loss, lymphadenopathy, lymphocytopenia, granulocytosis and wasting, in addition to high plasma levels of specific-immunoglobulin E (IgE) (Jungmann et al. 1996a,b). This condition is similar to scabies in humans, and is associated with high plasma levels of total immunoglobulin E (IgE) and IgG, as well as eosinophilia and elevated IL-4 production (Leung 2000; Walton et al. 2004).
Toxoplasma gondii infection is asymptomatic in most adult animals and humans due to the protective immunity developed by competent hosts (Frenkel 1988). In cases of immunodeficiency or during congenital infection, toxoplasmosis may emerge as a severe disease, which can cause host death (Denkers & Gazzinelli 1998).
Murine models of T. gondii infection have highlighted the important role performed by certain cytokines, such as interleukin-12 (IL-12), tumour necrosis factor-α (TNF-α), and interferon-γ (IFN-γ), as well as by the generation of reactive nitrogen intermediates (RNI) in the host resistance to early infection (Denkers & Gazzinelli 1998). After T. gondii infection, mice from virtually all strains develop a strong Th1 immune response, regardless of the fact that they have MHC haplotypes associated with susceptibility or resistance to toxoplasmosis (Gazzinelli et al. 1991, 1992). The resistance phenomenon in both acute and chronic infection with T. gondii in the murine model is highly dependent on endogenous IFN-γ (Suzuki et al. 1988, 2000; Scharton-Kersten et al. 1996; Denkers & Gazzinelli 1998). In the murine model of toxoplasmosis, C57BL/6 are considered more susceptible to T. gondii infection than BALB/c mice when inoculated with either the type I (C56) or the type II (ME-49) strain of the parasite (Suzuki et al. 1995, 2000). Both C57BL/6 (H-2b), and CBA (H-2k) mice are genetically susceptible to the development of toxoplasmic encephalitis (TE) (Suzuki et al. 1991, 1994; Brown et al. 1995). In contrast, genetically resistant strains [e.g. BALB/c (H-2d) mice] are able to control T. gondii infection in their brains, and they develop a latent chronic infection, as in the case of immunocompetent humans (Suzuki et al. 1991, 1994; Brown et al. 1995).
Considering that murine models greatly contribute to elucidating the immune mechanisms involved in T. gondii infection and the widespread prevalence of coinfections among human and animal hosts, there is growing interest in evaluating the influence of coinfection in the course of experimental toxoplasmosis. The interest is still greater when the coinfection is determined by an organism that triggers an immune response opposite to the Th1 type one induced by T. gondii, since type 1 and type 2 cytokines present cross-regulatory properties (Abbas et al. 1996). The influence of the Th2 immune response in proinflammatory immune mechanisms induced by T. gondii has been consistently studied in the susceptible lineage of mouse, C57BL/6 (Marshall et al. 1999; Araujo et al. 2001; Liesenfeld et al. 2004) and in all of the experimental procedures, the Th2 immune response altered the Th1 response induced by the protozoan. Concerning to this matter, recently, we have observed that C57BL/6 mice that had been previously infested with M. musculinus develop more severe manifestations of T. gondii infection than mono-infected mice (Welter et al. 2006). Related to BALB/c, in contrast to C57BL/6 mice, the Th2 immune response was not modified by the Th1 response induced by T. gondii infection (Liesenfeld et al. 2004).
To investigate whether the interference of the immune response in T. gondii infection is restricted to susceptible mice or whether it is extended to resistant animal strains, in this work, we have evaluated the outcome of toxoplasmosis in BALB/c mice that had been previously infested with M. musculinus. The infestation itself induced the production of Th2 cytokines, and coinfection with T. gondii was followed by accelerated mortality rates and high tissue protozoan burden, contrasting with what was observed for the BALB/c mice infected with T. gondii only. These results indicate that mice from a T. gondii-resistant lineage turned out to be highly susceptible hosts when previously infested with M. musculinus.
Materials and methods
Animals
Females BALB/c (H-2d) and Swiss (outbred) mice aged 8–12 weeks were bred as homozygotes and kept in the Bioterism Center of the Animal Experimentation Laboratory, Biomedical Sciences Institute, Federal University of Uberlândia, Brazil. The mice were housed under standard laboratory conditions. Animal experiments were conducted according to institutional guidelines for animal ethics.
Parasites and antigen preparations
The low-virulent ME-49 strain of T. gondii was maintained by intraperitoneal inoculation of the Swiss mice with brain homogenate. Myocoptes musculinus was obtained from and maintained in the Swiss mice infested by this ectoparasite in an isolated clean room with air filtration.
For T. gondii soluble antigen (STAg) preparation, tachyzoites of the RH strain were harvested from the peritoneal cavities of the Swiss mice that had been injected with 107 organisms 2 days earlier. The tachyzoites were sonicated and centrifuged, and the supernatant was collected and prepared as previously described (Gazzinelli et al. 1991).
For M. musculinus soluble antigen (SMAg) preparation, furs from infested mice were cut down and skin-scrapings were taken and placed in Petri dishes. The scrapings and furs were examined under stereomicroscope, and living mites of different stages were collected with a fine needle, placed in PBS and stored at −20 °C. The preparation of SMAg was carried out as described in (Kumar et al. 1998), with some modifications. After centrifugation of the collected material, the pellet was suspended in 5 mM borate buffered saline (pH 8.0) containing protease inhibitors, triturated in liquid nitrogen for mite disruption and extracted overnight at 4 °C under gentle shaking. After centrifugation at 45,000 × g for 30 min at 4 °C, the supernatant was concentrated and the protein concentration was determined.
Experimental procedure and tissue processing
Myocoptes musculinus infestations were accomplished by keeping the infested Swiss mice together with BALB/c mice. The infestation was monitored by periodically, taking skin scrapes, which were then examined under a microscope, as well as by clinical examination of the animals. BALB/c mice infested or not with M. musculinus 1 month earlier, were infected with 10 T. gondii cysts by intraperitoneal route, and groups of three mice were killed by cervical dislocation on days 0, 7, 14, 21 and 30 postinfection (p.i.). Blood samples were collected for serological analysis. Tissue samples, such as the central nervous system (CNS), lung, liver and spleen, were collected, fixed in 10% buffered formalin and processed routinely for paraffin embedding and sectioning. Tissue sections with 4 μm thickness (40 μm distance between sections) of each mouse were obtained in microtome and mounted on slides for histopathological and immunocytochemical studies. To verify the histological changes, tissue sections were stained with haematoxylin and eosin. In addition, the tissue sections were stained by the immunoperoxidase method for identification and quantification of tissue parasitism.
Quantification of tissue parasitism by immunocytochemistry
Tissue parasitism was quantified by immunocytochemistry, as described previously (Silva et al. 2002a). Deparaffinized sections were incubated for 30 min at 37 °C in 1% BSA and then overnight at 4 °C with polyclonal rabbit antibody, against antigens of the T. gondii ME-49 strain. Alternatively, tissue sections were also incubated with non-immune rabbit serum, which was used as negative control. After incubation with biotinylated sheep anti-rabbit antibodies, the sensitivity was improved with avidin–biotin complex (ABC kit, PK-4000; Vector Laboratories, Inc., Burlingame, CA, USA). The reaction was developed with H2O2 plus 3,3′-diaminobenzidine tetrahydrochloride (Amresco, Solon, OH, USA) for 5 min, and the slides were examined under optical microscopy.
Tissue parasitism was scored by counting the number of parasitophorous vacuoles per section in the CNS, or from 40 microscopic fields in the lung, liver and spleen (1 × 400), from two histological sections of each mouse and from three mice per group.
Blood eosinophil numbers and serum T. gondii or M. musculinus-specific IgE levels
Blood smears were air–dried on glass slides, stained by May Grunwald Giemsa (Merck, Darmstadt, Germany), and analysed for numbers of eosinophils. The relative numbers of eosinophils were expressed as percentage of white cells (eosinophils/100 white cells), and at least 300 cells were counted by blood smears.
Immunoglobulin E antibodies to T. gondii or M. musculinus were also measured by using an ELISA. Plates were coated with T. gondii (10 μg/ml) or M. musculinus (20 μg/ml) soluble antigens and subsequently incubated with serum samples diluted 1:2. Specific IgE antibodies were detected by incubation with biotinylated goat anti-mouse IgE (Caltag, Burlingame, CA, USA), followed by streptavidin-peroxidase (Sigma Co., St Louis, MO, USA). The reactions were revealed by adding the enzyme substrate consisting of 0.03% H2O2 and 0.01 M ABTS in 0.07 M citrate-phosphate buffer (pH 4.2).
Antibody levels were expressed as ELISA index (EI) as previously described (Ferro et al. 2002), and according to the following formula: EI = mean optical density (OD) of the sample/cutoff, where the cut off is calculated as the OD mean values of negative control sera plus three standard deviations. EI values > 1.0 were considered positive.
Cytokine quantification
BALB/c mice infested or not with M. musculinus and infected with T. gondii were killed on the 0th, 7th, 14th, and 21st days p.i. and their spleens were aseptically removed. The control groups consisted of one group of non-infected mice and another group infested with M. musculinus alone (30 days of M. musculinus infestation that corresponds to day zero of T. gondii infection). Cell suspensions were washed in RPMI medium, and erythrocytes were eliminated by hypotonic shock, then washed three times and adjusted to 5 × 106 cells/ml in RPMI medium supplemented by 10% heat-inactivated foetal calf serum. Cell suspensions were added (200 μl/well) to a 96-well tissue culture plate and incubated at 37 °C in 5% CO2 in the presence or absence of STAg (5 μg/ml) or concanavalin A (con A) (5 μg/ml) (Sigma). Cell-free supernatants were harvested and assayed for IFN-γ, following 72-h culture; IL-4, IL-5, and IL-10, following 48-h culture.
Interleukin-4, IL-5, IL-10 and IFN-γ were quantified by using an ELISA kit according to instructions from the manufacturer (BD PharMingen, San Diego, CA, USA, for IL-4, IL-5 and IL-10; and PeProtech, Veracruz, México, for IFN-γ). Cytokines levels were calculated by comparison with a standard curve constructed with recombinant murine cytokines [all from PharMingen except for recombinant IFN-γ (PeProtech)]. The detection sensitivity in these immunoassays was 200 pg/ml (IFN-γ), 7 pg/ml (IL-4), 15 pg/ml (IL-5) and 30 pg/ml (IL-10).
ELISA for measurement of T. gondii or M. musculinus-specific IgG1 and IgG2a antibodies
Plates were coated with T. gondii (10 μg/ml) or M. musculinus (20 μg/ml) soluble antigens and incubated with 1:10 dilutions of serum samples from individual animals. Peroxidase-labelled goat IgG anti-mouse IgG1 or IgG2a were subsequently added to the plates. The assay was developed by adding the enzyme substrate consisting of 0.03% H2O2 and 1,2-phenylendiamin (OPD; Merck, Schuchardt, Germany), and the OD was determined at 492 nm (Titertek Multiskan; Flow Laboratories, McLean, VA, USA). The antibody levels were arbitrarily expressed as EI as previously described. EI values >1.0 were considered positive.
Statistical analysis
The Kaplan–Meier method was used to compare the survival rates of the studied groups, as described by Fletcher et al. (1996). The survival curves were compared using logrank and chi-square tests that generate a two-tail P-value, to test the null hypothesis that they are identical in the overall groups of animals. Antibody and cytokine levels, as well as tissue parasitism and the numbers of eosinophils produced by different groups of animals, were compared by using Mann–Whitney U or Kruskal Wallis H tests, when appropriate. Statistical analyses and graphs were performed using GraphPad prism version 4.0 (GraphPad Software, San Diego, CA, USA). Values of P < 0.05 were considered statistically significant.
Results
Myocoptes musculinus infestation turned out BALB/c mice highly susceptible to T. gondii infection
BALB/c mice, known as a lineage that develops a chronic activation of Th2 lymphocytes in response to infestation with M. musculinus, became highly susceptible to T. gondii infection. Mice infested with M. musculinus only displayed progressive weight loss, achieving 18.3% on day 30 after infestation, in addition to erythematous and pruritic skin lesions as well as widespread fur loss. Weight values for adults started at 24 g and declined progressively to 11 g at the final phases of mange disease that occurred at around 13–15 months of age. Despite the wasting condition, BALB/c mice infested with M. musculinus only, presented a 100% survival rate during all experimental observation period (data not shown).
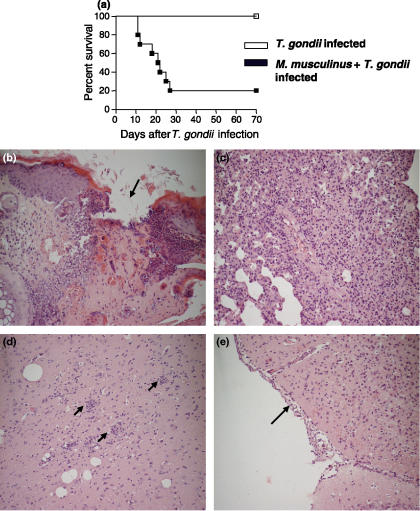
BALB/c mice, a commonly T. gondii-resistant lineage, presented a 100% survival rate at least 95 days after the T. gondii infection alone. However, when BALB/c mice were previously infested with M. musculinus and 30 days after were infected with T. gondii, these animals became highly susceptible to infection with the protozoan (χ2 = 10.87; P = 0.001; d.f. = 1), presenting 80% mortality 28 days post-T. gondii inoculation (Figure 1a). Additionally, double-infection worsened weight loss comparing with animals mono-infested with M. musculinus alone.
Figure 1.
Mortality rates and histological changes visualized by H&E staining in organs of BALB/c mice infected with Toxoplasma gondii plus Myocoptes musculinus or T. gondii alone. The survival of BALB/c mice (a) pre-infested with M. musculinus 30 days earlier and then infected by intraperitoneal route with 10 cysts of the ME-49 strain of T. gondii were monitored daily (10 animals per experimental group). The survival of coinfected mice was significantly reduced (χ2 = 10.87; P = 0.001; d.f. = 1) whether compared with mono-infected animals. Data are representative of at least two independent experiments that provided similar results. Skin sections from coinfected animals (day 15 of T. gondii infection) had an increased number of dermal infiltrates of inflammatory cells and an epithelial ulceration (arrow) (b). In the pulmonary tissue (day 15 of T. gondii infection), a severe mononuclear inflammatory infiltrate was seen in the septa (c). In the CNS on day 21 of T. gondii infection, glial nodules (arrows) (d) and infiltrates of inflammatory cells in the meninges (arrow) (e) were observed. Original magnification, ×20.
Concomitant M. musculinus infestation and T. gondii infection causes higher tissue parasitism and pathology if compared with mono-infected animals
In T. gondii mono-infected mice, major histopathological changes were observed in the lung on days 7 and 14, and in the CNS on day 21 p.i. In BALB/c double-infected mice, the major histopathological alterations were observed in the skin, lung and CNS, which presented more severe lesions.
The skin of M. musculinus-infested mice revealed an increased number of dermal infiltrates of mononuclear and polymorphonuclear leukocytes. In some areas, demarcated regions of epithelial ulcerations were observed (Figure 1b). Furthermore, cross-sections of parasites could be identified in the keratin layers of some animals.
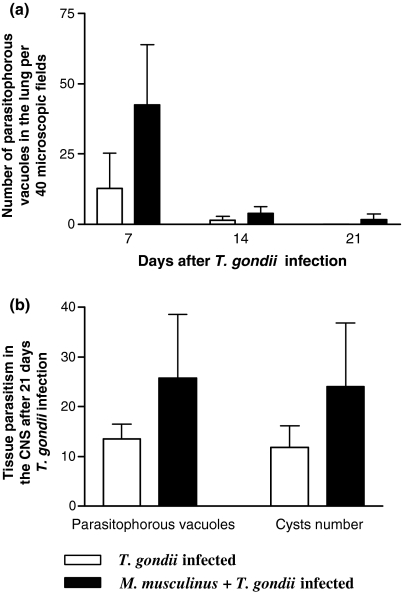
The lung of T. gondii-mono-infected mice presented interstitial pneumonia with mononuclear inflammatory infiltration in the alveolar septa. When coinfected, a severe mononuclear inflammatory infiltrate (Figure 1c) was observed in addition to a large number of polymorphonuclear cells. In some animals the granular leukocytes were associated with necrotic areas, leading to a focal suppurative interstitial inflammation. In parallel with the gravity of inflammatory changes, double-infected mice were more susceptible to higher T. gondii burden in the lung, mainly on day 7 p.i. (Figure 2a).
Figure 2.
Parasite load in the lung and CNS of BALB/c mice infected with Toxoplasma gondii alone or coinfected with Myocoptes musculinus. Tissue parasitism was detected by immunocytochemistry staining and scored by counting the number of parasitophorous vacuoles and cysts per section in the CNS and in 40 microscopic fields in the lung. The coinfected animals were more susceptible to higher parasite burden in the lung (a) and in the CNS (b) whether compared with mono-infected animals. Similar results were obtained in two independent experiments and data are expressed as mean and standard deviation.
In the CNS, the lesions were characterized by diffuse infiltrates of mononuclear cells, glial nodules (Figure 1d), vascular cuffing by lymphocytes, and focal infiltrates of inflammatory cells in the meninges (Figure 1e) on day 21 p.i.. Also in the CNS, pre-infestation with M. musculinus contributed to higher tissue parasitism by T. gondii whether compared with mono-infected mice (Figure 2b).
Myocoptes musculinus infestation interferes with T. gondii-specific IgG1 and IgG2a isotypes
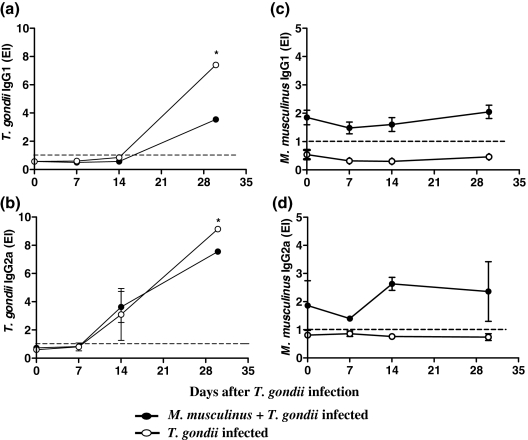
In serum samples from both mono and double-infected mice, T. gondii-specific IgG1 and IgG2a antibodies were detected after the 14th and 7th day p.i., respectively, showing an increasing seropositivity profile (Figure 3a,b). T. gondii-specific IgG1 and IgG2a levels were higher in mono- than in double-infected mice on day 30 p.i. (Mann–Whitney U = 0.0; P = 0.0022; d.f. = 2) (Figures 3a,b).
Figure 3.
Kinetics of Toxoplasma gondii- or Myocoptes musculinus-specific IgG1 and IgG2a isotypes in serum samples from mono- or double-infected BALB/c mice. The animals were experimentally infested or not with M. musculinus 30 days prior infection with 10 cysts of the T. gondii ME-49 strain. On days 0, 7, 14 and 30 after T. gondii infection, serum levels of T. gondii-specific IgG1 (a), IgG2a (b), or M. musculinus-specific IgG1 (c) and IgG2a (d) were measured by ELISA. Mice infected with T. gondii alone presented significantly higher specific IgG1 and IgG2a levels on day 30 after T. gondii inoculation if compared with coinfected mice. Results are expressed as ELISA index (EI), where values of EI > 1.0 (horizontal dashed line) were considered positive. Data represent the mean and standard deviation obtained from three mice per each time point and are representative of at least two independent experiments that provided similar results. *Significantly different from values obtained for double-infected mice (Mann–Whitney U = 0.0; P = 0.0022; d.f. = 2).
The detection of M. musculinus-specific IgG1 and IgG2a antibodies revealed a significant production of both isotypes after 30 days of infestation by the mite alone (day 0 of T. gondii infection) and IgG1 levels were not affected by T. gondii infection (Figure 3c). The highest level of M. musculinus-specific IgG2a was observed on day 14 after T. gondii infection (Figure 3d).
Production of cytokine by spleen cells from mono- or double-infected mice
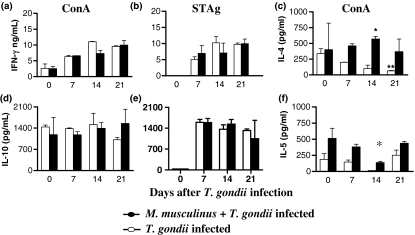
To verify the influence of M. musculinus infestation in the Th1 immune response normally induced by toxoplasmosis, the cytokine production was measured in the supernatant of spleen cells from mono- and double-infected mice. T. gondii infection induced rising IFN-γ levels from spleen cells stimulated in vitro with STAg until 21 days p.i. (Figure 4b). After in vitro stimulation with Con A, similar IFN-γ concentrations were obtained (Figure 4a). When coinfected animals were analysed, the levels of IFN-γ production were lower on day 14 p.i. (Figure 4a, b).
Figure 4.
Concentration of interferon-γ (IFN-γ), interleukin (IL)-4, IL-5 and, IL-10 in the supernatants of spleen cells taken from BALB/c mice mono- or double-infected with Myocoptes musculinus and Toxoplasma gondii. Spleen cells from mice infested or not with M. musculinus 30 days prior infection with 10 cysts of T. gondii were harvested on days 0, 7, 14 and 21 after T. gondii infection and cultured in the presence of soluble tachyzoite antigen (STAg; 5 μg/ml) (b,e) or Concanavalin A (Con A; 5 μg/ml) (a,c,d,f). Supernatants were collected and assayed by ELISA. Data represent the mean and standard deviation of three mice per time point and are representative of at least two independent experiments that provided similar results. *Significantly different from values obtained for animals mono-infected with T. gondii (Kruskal–Wallis test, H = 10.38; P = 0.0156; d.f. = 2). **Significantly different from values obtained for non-infected mice (Kruskal–Wallis test, H = 9.667; P = 0.0216; d.f. = 2).
Type 2 cytokines, IL-4, IL-5 and IL-10 in single and double-infections were also analysed (Figure 4c–f). Important IL-4 production in the supernatant of spleen cells stimulated with Con A was observed in mono-infected mice (day 0 of T. gondii infection) (Figure 4c). However, consistently with the Th1 response induced by T. gondii when this mouse lineage was infected with the protozoan, a significant drop in the production of this cytokine was verified, particularly on day 21 p.i. (Kruskal–Wallis test, H = 9.667; P = 0.0216; d.f. = 2) (Figure 4c). Furthermore, when mice were coinfected, M. musculinus induced significantly higher levels of IL-4 production on day 14 p.i. than those found in animals infected with T. gondii alone (Kruskal–Wallis test, H = 10.38; P = 0.0156; d.f. = 2).
Concerning IL-5, M. musculinus infestation for 30 days induced significant levels of this cytokine (day 0 of T. gondii infection). In addition, double-infection also induced increased levels of this cytokine (Figure 4f). Stimulation with STAg did not induce detectable IL-4 and IL-5 levels in either mono- or double-infected mice (data not shown), showing that the production of these type 2 cytokines was probably induced by the acarian and not by the protozoan.
Interleukin-10 was detected in both mono- and double-infected mice, showing that the double-infection did not alter IL-10 production (Figure 4d,e). T. gondii infection induced higher IL-10 levels in spleen cells on day 7 p.i., in mono- and double-infected mice under STAg stimulation (Figure 4e).
Our results showed that M. musculinus infestation decreased IFN-γ production on day 14 after T. gondii infection, which corresponds to the peak of IL-4 levels. Conversely, M. musculinus infestation significantly increased the levels of IL-4 and IL-5 cytokines after infection with the protozoan, probably impairing the effective mechanisms that control T. gondii replication.
Myocoptes musculinus infestation increases markers of Th2 response
Blood eosinophilia was also analysed under the influence of M. musculinus infestation. As shown in Table 1, BALB/c mice infested 30 days earlier with M. musculinus only (day 0 of T. gondii infection) showed a significantly larger number of blood eosinophils when compared with non-infested mice (Mann–Whitney U = 4.0; P = 0.0140; d.f. = 2). Coinfected animals, however, presented a remarkable reduction in the number of blood eosinophils when compared with mice that were infested with the acarian only. Animals that were mono-infected with T. gondii only showed a small number of eosinophils throughout the period of observation. Thus, despite being disturbed by M. musculinus infestation, the immune response induced by T. gondii interfered with the number of this cell phenotype.
Table 1.
Number of eosinophils in peripheral blood smears of mono- or double infected BALB/c micea
| Number of eosinophils/100 white cellsb | ||||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | |
| Naive | 0.6 ± 0.3 | – | – | – |
| Mono-infected with T. gondii | – | 0.8 ± 0.7 | 0.8 ± 0.9 | 0.7 ± 0.3 |
| Mono-infected with M. musculinus | 2.5 ± 0.6* | – | – | – |
| Double infected with M. musculinus and T. gondii | – | 1.2 ± 0.2 | 1.0 ± 0.5 | 1.4 ± 0.3 |
The animals were infested or not with M. musculinus 30 days prior infection with 10 cysts of T. gondii. On days 0, 7, 14, and 21 after T. gondii infection, blood was obtained and blood smears were prepared and analysed following May Grünwald staining.
A total of 300 white cells were counted per mouse at a magnification of ×400 and the number of eosinophils per 100 white cells was determined. Data represent the mean and standard deviation from four mice per group and are representative of at least two independent experiments that provided similar results.
Significantly different from values obtained for mono-infected mice with T. gondii (Mann–Whitney U = 4.0; P = 0.0140; d.f. = 2).
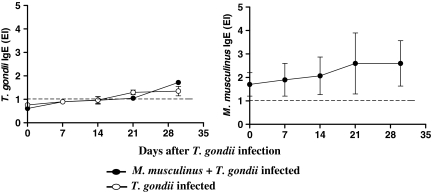
Toxoplasma gondii-specific IgE antibodies were analysed during T. gondii infection in mono- or double-infected animals. The IgE isotype was detected in low levels starting from day 21 after infection with the protozoan, and there was no difference in the levels of this isotype between mono- and double-infected mice (Figure 5a).
Figure 5.
Kinetics of Toxoplasma gondii- or Myocoptes musculinus-specific IgE isotype in serum samples from mono- or double-infected BALB/c mice. The animals were experimentally infested or not with M. musculinus 30 days prior infection with 10 cysts of the T. gondii ME-49 strain. On days 0, 7, 14 and 30 after T. gondii infection, serum levels of T. gondii-specific IgE (a) or M. musculinus-specific IgE (b) were measured by ELISA. Results are expressed as ELISA index (EI), where values of EI > 1.0 (horizontal dashed line) were considered positive. Data represent the mean and standard deviation obtained from three mice per each time point and are representative of at least two independent experiments that provided similar results.
To verify whether T. gondii infection interferes with the levels of M. musculinus-specific IgE, we measured the levels of this isotype during T. gondii infection. Mice infested with M. musculinus showed increases in a typical Th2 marker, elevated serum IgE levels (Figure 5b) that were not significantly affected by the subsequent T. gondii infection. In addition, M. musculinus-specific IgE levels were higher than T. gondii-specific IgE levels.
Discussion
In the present study, we have shown that BALB/c mice, a lineage previously resistant to T. gondii infection, turned out to be highly susceptible to this protozoan when pre-infested with the acarian parasite M. musculinus. Accelerated death and high rates of parasite burden in the lung and CNS were observed in BALB/c mice infested with M. musculinus and infected with T. gondii. Thus, animals with mange disease have their immune response altered, and it worsened the outcome of T. gondii infection. Disease due to M. musculinus started 1 month after the acarian infestation, and it was characterized by reddening of the skin, pruritus, ruffled fur, hair loss and progressive weight loss. Our own observations support the role of the parasite itself as a primary cause of lesions. As previously reported, the fact that the mites do not invade the tissue also supports a hypersensitive reaction to parasite-derived antigens, which may enter the body through skin lesions (Jungmann et al. 1996a). The early mortality observed in double-infected mice is probably due to the major pathological findings detected mainly in the pulmonary tissue and in the CNS as a result of the T. gondii infection, in addition to high parasite burden in these organs, associated with a wasting syndrome induced by the acarian infestation. According to our previous investigations using C57BL/6 mice (Silva et al. 2002a,b), the lung and CNS are important sites of T. gondii replication and morbidity in the acute and chronic phases of infection, respectively. In the present study, however, we have observed that this condition is worsened when BALB/c mice have their Th1 response impaired because of the previous infection with M. musculinus. In contrast with the induction of Th2-like immune mechanisms in BALB/c and C57BL/6 mice by M. musculinus (Jungmann et al. 1996b; Welter et al. 2006), a dominant Th1 immune response was induced in T. gondii infection (Denkers & Gazzinelli 1998). In previous studies using BALB/c-background IFN-γ-deficient (IFN-γ−/−) mice, which are highly susceptible to T. gondii infection, it had been demonstrated that the protective activity to control the protozoan is mediated by non-cytolitic and IFN-γ dependent Th1 mechanisms in the CNS (Wang et al. 2004).
We have therefore hypothesized that the Th2 immune response developed during mite infestation could be detrimental to the resistant host to control T. gondii infection. In our experimental design, we observed that the immune mechanisms induced by M. musculinus slightly alter the levels of IFN-γ production, and lower levels of this cytokine were observed only on day 14 after T. gondii infection. In contrast, throughout the observation period, increased IL-4 levels were observed in the supernatants of spleen cells following stimulation with Con A in coinfected animals. The increase in IL-4 concentrations appeared to be M. musculinus-specific, since in vitro stimulation of spleen cells with STAg did not result in the production of this cytokine. In addition, these results were associated with increased eosinophilia and considerable production of specific IgE against M. musculinus, which is not changed due to T. gondii inoculation in coinfected animals. These data suggest that the production of Th2 cytokines induced by M. musculinus infestation in BALB/c mice could negatively interfere with the effector mechanisms that control T. gondii replication.
In vitro experiments have shown a crucial role for both IFN-γ and TNF-α in the induction of RNI and microbicidal activity displayed by murine macrophages against tachyzoites (Langermans et al. 1992). It is well known that IL-4 antagonizes the macrophage-activating effects of IFN-γ, thus inhibiting cell-mediated immune reaction (Gajewski & Fitch 1988). Therefore, the higher levels of IL-4 found in supernatants of spleen cells from coinfected BALB/c mice stimulated with Con A, suggest that these IL-4 levels in vivo could be impairing the macrophage activation state.
When we analysed T. gondii-specific IgG isotypes, we observed that the coinfected mice had lower T. gondii-specific IgG1 and IgG2a antibody levels than the mono-infected mice. Since released tachyzoites can be effectively killed, at least in part, through antibody-mediated phagocytosis by macrophages (Anderson et al. 1976), the lower antibody levels could be contributing to the higher parasite burden verified in coinfected mice. On the other hand, T. gondii infection did not alter the levels of M. musculinus-specific IgG isotypes.
Considering the high prevalence of T. gondii infection (Dubey 2004) and ectoparasite infestation (Walton et al. 2004) in many countries and that M. musculinus and T. gondii could infect hosts simultaneously, we investigated the influence of coinfection in the immune response of a T. gondii-resistant mouse lineage. Immunocompetent humans are usually resistant to the development of TE, the same happening to genetically resistant mouse strains. Thus, the results of the present study raised the hypothesis that if coinfection with these two unrelated parasites occurs naturally in humans, the outcome of this dual infection could impair control of the T. gondii infection in these hosts, in a similar way to the condition described herein for BALB/c mice.
It was previously demonstrated that the downregulation of established Th2 response by T. gondii infection was dependent on the genetic background of the mice. In this context, the Th2 immune response induced by previous infection with Nippostrongylus brasiliensis is modified by T. gondii infection in C57BL/6 but not in BALB/c mice (Liesenfeld et al. 2004). In contrast, in our present investigation using a resistant lineage of mouse to T. gondii infection the acarian itself was able to impair the Th1 immune response normally induced by T. gondii. In other coinfection experimental models using BALB/c mice, infection with T. gondii before infection with Leishmania major protected the L. major susceptible BALB/c mice (Santiago et al. 1999). Infection with T. gondii posterior infection with Helicobacter felis induced mucosal IFN-γ and IL-12 elevated levels and IL-10 substantially reduced becoming BALB/c mice susceptible to T. gondii infection associated with severe mucosal inflammation (Stoicov et al. 2004). In our present work, we demonstrated for the first time that the immune response to the acarian M. musculinus interferes with the Th1 response normally induced by the protozoan T. gondii in a resistant mouse lineage.
In conclusion, the results presented herein suggest that the immune response induced by the mange interferes with the type-1 immune response induced by T. gondii and with the effector mechanisms that control protozoan burden. Furthermore, we demonstrated that these alterations are detrimental to a host that is normally known to be resistant to this parasite.
Acknowledgments
This work was supported by Conselho Nacional de Pesquisa Científica e Tecnológica (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We thank Dr Ana Maria Aparecida Guaraldo, Laboratory of Parasitology from the University of Campinas, who helped us with her entomological expertise.
References
- Abbas AK, Murphy K, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Jr, Bautista SC, Remington JS. Specific antibody-dependent killing of Toxoplasma gondii by normal macrophages. Clin. Exp. Immunol. 1976;26:375–380. [PMC free article] [PubMed] [Google Scholar]
- Araujo MI, Bliss SK, Suzuki Y, Alcaraz A, Denkers EY, Pearce EJ. Interleukin-12 promotes pathologic liver changes and death in mice coinfected with Schistosoma mansoni and Toxoplasma gondii. Infect. Immun. 2001;69:1454–1462. doi: 10.1128/IAI.69.3.1454-1462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR, Hunter CA, Estes RG, et al. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- Dawson DV, Whitmore SP, Bresnahan JF. Genetic control of susceptibility to mite-associated ulcerative dermatitis. Lab. Anim. Sci. 1986;36:262–267. [PubMed] [Google Scholar]
- Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis – a waterborne zoonosis. Vet. Parasitol. 2004;126:57–72. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Ferro EAV, Silva DAO, Bevilacqua E, Mineo JR. Effect of Toxoplasma gondii infection kinetics on trophoblast cell population in Calomys callosus, a model of congenital toxoplasmosis. Infect. Immun. 2002;70:7089–7094. doi: 10.1128/IAI.70.12.7089-7094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RH, Fletcher SW, Wagner EH. Clinical Epidemiology: The Essentials. 3. Baltimore, MD: Willians and Wilkins; 1996. [Google Scholar]
- Frenkel JK. Pathophysiology of toxoplasmosis. Parasitol. Today. 1988;4:273–278. doi: 10.1016/0169-4758(88)90018-x. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Fitch FW. Anti-proliferative effect of IFN-γ in immunoregulation. 1. IFN-γ inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-γ production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- Gazzinelli RT, Hu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactive chronic infection with Toxoplasma gondii. J. Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- Jungmann P, Guénet JP, Cazenave PA, Coutinho A, Huerre M. Murine acariasis: I. Pathological and clinical evidence suggesting cutaneous allergy and wasting syndrome in BALB/c mouse. Res. Immunol. 1996a;147:27–38. doi: 10.1016/0923-2494(96)81546-x. [DOI] [PubMed] [Google Scholar]
- Jungmann P, Freitas A, Bandeira A, et al. Murine acariasis: II. Immunological dysfunction and evidence for chronic activation of Th-2 lymphocytes. Scand. J. Immunol. 1996b;43:604–612. doi: 10.1046/j.1365-3083.1996.d01-259.x. [DOI] [PubMed] [Google Scholar]
- Kumar L, Sridhara S, Singh BP, Gangal SV. Characterization of cogon grass (Imperata cilindrica) pollen extract and preliminary analysis of grass group 1, 4 and 5 homologues using monoclonal antibodies to Phleum pretense. Int. Arch. Allergy Immunol. 1998;117:174–179. doi: 10.1159/000024007. [DOI] [PubMed] [Google Scholar]
- Langermans JA, Van Der Hulst MEB, Nibbering PH, Hiemstra PS, Fransen L, Van Furth R. IFN-γ induce L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-α. J. Immunol. 1992;148:568–574. [PubMed] [Google Scholar]
- Leung DY. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J. Allergy Clin. Immunol. 2000;105:860–876. doi: 10.1067/mai.2000.106484. [DOI] [PubMed] [Google Scholar]
- Liesenfeld O, Dunay IR, Erb KJ. Infection with Toxoplasma gondii reduces established and developing Th2 responses induced by Nippostrongylus brasiliensis infection. Infect. Immun. 2004;72:3812–3822. doi: 10.1128/IAI.72.7.3812-3822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AJ, Brunet LR, Van Gessel Y, et al. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-α and early death in C57BL/6 mice. J. Immunol. 1999;163:2089–2097. [PubMed] [Google Scholar]
- Matsuda H, Watanabe N, Geba GP, et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997;9:461–466. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- Santiago HC, Oliveira MAP, Bambirra EA, et al. Coinfection with Toxoplasma gondii inhibits antigen-specific Th2 immune responses, tissue inflammation, and parasitism in BALB/c mice infected with Leishmania major. Infect. Immun. 1999;67:4939–4944. doi: 10.1128/iai.67.9.4939-4944.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharton-Kersten TM, Wynn TA, Denkers EY, et al. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- Silva NM, Rodrigues CV, Santoro MM, Reis LFL, Alvarez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous IFN-γ and requirement of IRF-1. Infect. Immun. 2002a;70:859–868. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NM, Tafuri WL, Alvarez-Leite JI, Mineo JR, Gazzinelli RT. Toxoplasma gondii: in vivo expression of BAG-5 and cyst formation is independent of TNF p55 receptor and inducible nitric oxide synthase functions. Microbes Infect. 2002b;4:261–270. doi: 10.1016/s1286-4579(02)01537-x. [DOI] [PubMed] [Google Scholar]
- Stoicov C, Whary M, Rogers AB, et al. Coinfection modulates inflammatory responses and clinical outcome of Helicobacter felis and Toxoplasma gondii infections. J. Immunol. 2004;173:3329–3336. doi: 10.4049/jimmunol.173.5.3329. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Joh K, Orellana MA, Conley FK, Remington JS. A gene (s) within H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology. 1991;74:732–739. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Joh K, Kwon OC, Yang Q, Conley FK, Remington JS. MHC class I gene (s) in the D/L region but not the TNF-a gene determines development of toxoplasmic encephalitis in mice. J. Immunol. 1994;153:4649–4654. [PubMed] [Google Scholar]
- Suzuki Y, Yang Q, Remington JS. Genetic resistance against acute toxoplasmosis depends on the strain of Toxoplasma gondii. J. Parasitol. 1995;81:1032–1034. [PubMed] [Google Scholar]
- Suzuki Y, Kang H, Parmley S, Lin S, Park D. Induction of tumor necrosis factor-α and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-γ in genetically resistant BALB/c mice. Microbes Infect. 2000;5:455–462. doi: 10.1016/s1286-4579(00)00318-x. [DOI] [PubMed] [Google Scholar]
- Walton SF, Holt DC, Currie BJ, Kemp DJ. Scabies: new future for a neglected disease. Adv. Parasitol. 2004;57:309–376. doi: 10.1016/S0065-308X(04)57005-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Kang H, Kikuchi T, Suzuki Y. Gamma interferon production, but not perforin-mediated cytolytic activity, of T cells is required for prevention of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. Infect. Immun. 2004;72:4432–4438. doi: 10.1128/IAI.72.8.4432-4438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter A, Mineo JR, Silva DAO, et al. An opposite role is exerted by the acarian Myocoptes musculinus in the outcome of Toxoplasma gondii infection according to the route of the protozoa inoculation. Microbes Infect. 2006;8:2618–2628. doi: 10.1016/j.micinf.2006.07.006. [DOI] [PubMed] [Google Scholar]