Abstract
Clonal histotropism and biological characters of five clones isolated during the early acute phase of the infection of Swiss mice with the Colombian strain of Trypanosoma cruzi (T. cruzi I), Biodeme Type III, were investigated. Clones were isolated from mice at the 10th and the 30th day of infection with the Colombian strain. Isolation was performed by micromanipulation and injection of one trypomatigote blood form into newborn mice, followed by passages into suckling mice for obtaining the inocula for the experimental groups. Mice infected with parental strain were also studied. All the clones have shown the basic characteristics of Biodeme Type III, with the same patterns of parasitemia, tissue tropism, morphological characters and isoenzymic profiles, such as the parental strain. Histotropism was most intense to myocardium and skeletal muscles, with intense lesions found in the advanced phase (20th to 30th day of infection). Both parental strain and the clones were seen to parasitize several organs and tissues; amastigote nests were identified in the cytoplasm of macrophages, adipose cells, smooth muscle of intestinal wall and Auerbach's neuronal plexus. The findings of the present study confirm the homology of the clones isolated from the Colombian strain, with predominance of a ‘principal clone’ and an ubiquitous distribution of parasites belonging to a same clone.
Keywords: Biodeme type III, clonal histotropism, clones of T. cruzi, Colombian strain, Trypanosoma cruzi
Strains of Trypanosoma cruzi are multiclonal populations that can be grouped into few discrete types, differing in pathogenicity and virulence, as well as in histotropism and response to chemotherapy. These characteristics are correlated with the grouping of T. cruzi strains into the different Biodemes Types I, II and III (Andrade 1974; Andrade & Magalhães 1997) and Zymodemes Z1, Z2 and Z3 (Miles et al. 1980), now included as groups: T. cruzi I and T. cruzi II (Momen 1999). Tissue tropism is an intrinsic characteristic of strains of T. cruzi of the three biodemes and, probably, results from their clonal composition. Several studies on the clonal composition of T. cruzi have been performed based on biological and isoenzymic characteristics, showing either a clonal homogeneity (Dvorak et al. 1980; Goldberg & Silva 1983; Gomes et al. 1991) or heterogeneity (Engel et al. 1982; Marques & Chiari 1988; Lima et al. 1990). In previous investigations (Camandaroba et al. 2001), it has been shown that clones isolated from the Colombian strain at the peak of parasitemia (30th day of infection) disclosed phenotypic homogeneity and high similarity with the parental strain, high resistance to chemotherapy (Camandaroba et al. 2003) as well as the same histotropism (Camandaroba et al. 2001). Although disclosing a similarity in the phenotypic behaviours, different degrees of virulence were found between the clones. The most virulent clones were identified by early and higher parasitemic levels and high mortality. The same clone presents slender and broad forms in the course of infection, with variations in their percentages; probably an increased number of slender forms in the early phase of infection (14th to 20 days of infection), seen in some isolated clones, results from an early and high multiplication of the parasite. The genetic characterization of the clones has been performed by the schizodeme method (Morel et al. 1980), by restriction length polymorphism (RFLP) analysis of kinetoplast DNA (kDNA), after amplification by polymerase chain reaction (PCR) of the fragments of kDNA minicircles of T. cruzi (Camandaroba et al. submitted to publication) and have disclosed genotypical homogeneity of these clones.
The parasites originated from the multiplication of one clone in the vertebrate model, can be distributed through several organs and tissues. These observations are not keeping with the recently proposed theory of a ‘clonal histotropic model’ (Macedo & Pena 1998), which suggested that each clone of one strain is destined to one specific tissue. These studies were based on the molecular characterization of the clones using the ‘low stringence single specific primer’ (LSSP-PCR) method. The ‘excluding presence’ of one genetic population, either in the myocardium or in the digestive tract, was described in several studies (Vago et al. 1996a, b, 2000). In the present investigation, a detailed study on the biological behaviour and the histotropism of clones of the Colombian strain, isolated in the 10th day of infection, as well as of two clones isolated in the 30th day, has been performed by direct search of intracellular amastigotes in several organs and tissues by histopathological and immuno-histochemical methods. Results have indicated that cloned populations, isolated by micromanipulation (Dvorak 1985), from one single trypomastigote blood form, showed a disseminated distribution, combined with a preferential tropism for skeletal muscles, reproducing the behaviour of the parental strain, in which parasitism of multiple organs has also been described.
These results confirm the hypothesis of predominance of a ‘principal clone’ (Andrade 1999), in those strains that show clonal homology at the phenotypic and genetic levels, as previously demonstrated with the clones isolated from the 21SF strain (Campos & Andrade 1996, Campos et al. 1999).
Materials and methods
Parental strains and clones of T. cruzi strains
For the present investigation, five clones of the Colombian strain, isolated in the 10th day of infection (Col-C8, Col-C10, Col-C13, Col- C14 and Col-C15), and two clones previously isolated (Camandaroba et al. 2001) in the 30th day of infection (Col-C1 and Col-C5) have been used. The clones were obtained by micromanipulation (Dvorak 1985), by isolating one single parasite, from peripheral blood of mice infected with the Colombian strain of T. cruzi (parental strain). Citrated blood, collected from infected mice, was centrifuged in a refrigerated centrifuge at 900 g, for separation of erythrocytes and leucocytes; the supernatant was examined in a Neubauer chamber for evaluation of parasite concentration. Dilution was performed with PBS, pH 7.2, and drops of 1 µl were distributed in the wells of titration plaques and examined on an inverted microscope. By micromanipulation, a single trypomastigote form was isolated with 1-ml syringe and inoculated into one 8-day-old mouse. From positive animals, successive passages into suckling mice were performed for each clone. Clones were maintained by cryopreservation into liquid nitrogen until re-inoculation into groups of suckling mice for obtaining the inocula for the groups of study. The parental strain was maintained in laboratory by successive passage into mice and inoculated into the study group.
The clones isolated by the 10th day of infection were submitted to biological characterization through the infection of mice, by the evolution of parasitemia on the 7th to 50th day after infection, by the cumulative mortality and isoenzymic profiles of parasites isolated through haemoculture.
Histotropism
It was investigated by examination of several organs and tissues: heart, skeletal muscle, intestinal tract, liver, spleen, spinal cord and sympathetic ganglia.
As control, the parental Colombian strain was studied under the same methods.
Experimental groups
For the parental strain and each clone, one group of 25 Swiss mice weighing 10–15 g was used, in a total of eight experimental groups. One control group was inoculated with the parental strain Col-P.
Inoculum
1 × 105 trypomastigotes blood forms obtained from mice inoculated with each clone or the parental strain were inoculated intraperitoneally into the animals of each experimental group.
Biological parameters
Parasitemic profiles, morphology of parasites in peripheral blood, in the early infection, and cumulative mortality were evaluated in the several groups inoculated with the clones isolated at the 10th day of infection. The clones isolated at the 30th day of infection have been previously characterized under the same parameters (Camandaroba et al. 2001). Parasitemia was evaluated in five animals from the 7th to the 50th day of infection and expressed as the means obtained in each day. Cumulative mortality was evaluated from the 30–50th day of infection.
Morphology
Evaluation of the percentage of slender and broad forms of parasites in peripheral blood during the course of infection was performed in blood smears stained with May Grünwald-Giemsa staining method.
Isoenzymic characterization
It was performed according to the method previously described (Miles et al. 1980; Andrade et al. 1983); briefly, parasites from culture in Warren medium were washed four times, with centrifugation at 2000 g in buffer tris-KRT, pH 7.3, and enzymic extracts were prepared and stored into liquid nitrogen as ‘pearls’. The following isoenzymes were tested: alanine aminotransferase (ALAT-E.C.2.6.1.2); aspartate aminotransferase (ASAT-E.C.2.6.1.1); glucose-phosphate isomerase (GPI-E.C.5.3.1.9) and phospho-glucomutase (PGM-E.C.2.7.5.1). Electrophoresis was performed according to the method of Miles et al. (1980).
Statistical analysis
Means of parasitemia were evaluated between the parental strain and clones in the early phase (12th to 14th days), in the intermediary phase (17th to 19th days) and in the late phase of acute infection (25th day) by the comparative nonparametric Mann-Witney test, using the Biostat program.
For comparative evaluation of the mortality of mice infected with the parental strain and clones, a nonparametric Fisher test was applied, using the program Epi info 6.
The predominance of slender or broad forms of the parasite in peripheral blood of mice infected with the parental strain and its clones was evaluated in the 14th and 20th days postinfection by the nonparametric test (X2 chi-square test). For several tests, the signficance level was of P = 0.05.
Histotropism
From each experimental group, three mice were sacrificed in the 10th, 14th, 20th, 25th and 30th day postinfection. Complete necropsies were performed, and fragments of the myocardium, skeletal muscle, oesophagus, intestinal tract, spleen and liver were formalin fixed, paraffin embedded and sections stained with haematoxylin and eosin. For the identification and study of the sympathetic ganglia and of the spinal cord, section of the vertebral column at the lombar region was taken. For that, two longitudinal sections were made 0.5 cm away from the vertebral lateral apophyses on each side, and two horizontal cuts as previously described (Souza et al. 1996). After fixation in 10% formalin, the tissues having the vertebra with the spinal cord in its centre were cut into two to three blocks, decalcified in 5% EDTA and then divided longitudinally, embedded in paraffin and sections stained with haematoxylin and eosin.
Immunohistochemistry for immunolabelling of T. cruzi forms
Paraffin sections were treated with purified, specific anti-T. cruzi IgG produced in rabbits in the dilution of 1 : 300 overnight at 4 °C. After three washes with PBS, the tissues were incubated with 1 : 20 dilution of goat normal serum, followed by incubation with peroxidase-conjugated goat anti-rabbit IgG antibody (DAKO P448) in the dilution of 1 : 800 during 30 min at 37 °C. The colour was developed with 0.6 mg/µl 3,3′-diaminobenzidine tetrahydrochloride (DAB) + 0.012% DMSO and 0.001% H2O2 at room temperature. Sections were counterstained with 1% methyl-green for 15 min, dehydrated and mounted in Canadian balsam.
Results
Parasitemia, cumulative mortality and morphology of parasites
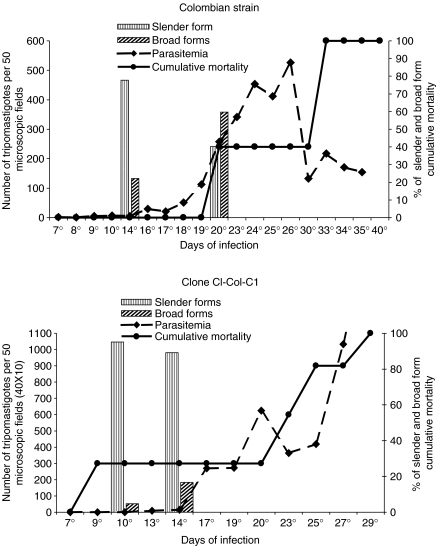
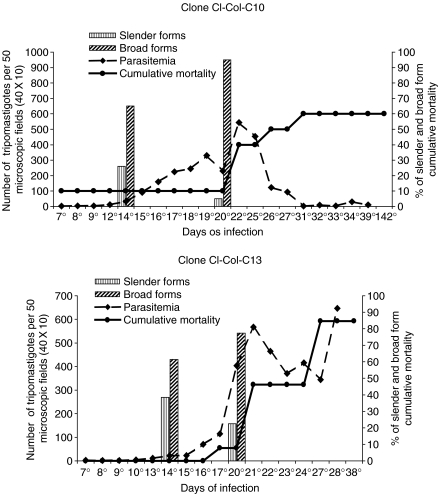
Parasitemia, cumulative mortality and morphology of parasites are expressed in the Figures 1 and 2 for the parental strain and clones C-10 and C-13.
Figure 1.
Profiles of parasitemia, cumulative mortality and morphology of parasites in peripheral blood from mice infected with the parental strain (P-Col) and with the clone Col-C1 showing high peaks of parasitemia at the 26th and 29th days of infection, respectively, 100% of mortality until the 40th day and predominance of slender parasite forms in the peripheral blood in an early phase of infection (10th day).
Figure 2.
Two examples of clones isolated from the Colombian strain: Clone Col-C10 showing 60% of mortality and parasitemic peak at 22 days that decreases progressively; Clone Col-C13, higher peaks of parasitemia in the 22th day, increasing until the 28th day, and 80% mortality until the 38th day. Both clones showed a predominance of broad forms of the parasite in the peripheral blood.
Parasitemic peaks varied between the 27th to the 37th day for the parental strain and clones Col-C1, Col-C5, Col-C8, Col-C13 and Col-C15. Earlier peaks occurred with the clones Col-C10 (22th day), Col-C13 (21th day) and Col-C14 (17th day).
Statistical analysis
Comparative analysis of the parasitemic levels between the parental strain and its clones, by the Mann–Whitney nonparametric test, did not reveal statistically significant differences for the clones Col-C1, Col-C8, Col-C10, Col-C13 and Col-C15 in the early, intermediate or late phases. Significant differences were seen for the clones Col-C5 (P = 0.04 in the intermediary and P = 0.04 in the late phases) and Col-C14 (P = 0.09 in the early phase) (Table 2).
Table 2.
Statistical analysis of the cumulative mortality in mice infected with Colombian strain and its clones by Fisher exact nonparametric test
| Strain vs. clones | Mortality in mice | Percentage (%) | P value |
|---|---|---|---|
| ColP vs. Col-C1 | 9/9–11/11 | 100–100 | P = 0.7 |
| ColP vs. Col-C5 | 9/9–1/13 | 100–7.6 | P = 0.01 |
| ColP vs. Col-C8 | 4/10–0/10 | 40–0 | P = 0.01 |
| ColP vs. Col-C10 | 4/10–5/10 | 40–50 | P = 0.01 |
| ColP vs. Col-C13 | 4/10–11/13 | 40–84.6 | P = 0.00005 |
| ColP vs. Col-C14 | 4/10–9/14 | 40–64.2 | P = 0.001 |
| ColP vs. Col-C15 | 4/10–4/10 | 40–40 | P = 0.2 |
Col-C: clones; Col-P, Colombian Parental strain.
Morphological aspects
The majority of the clones showed a predominance of broad forms from the 14th day of infection. However, predominance of slender forms in the 14th day of infection (75%) was detected in the parental strain (Col-P), and a significant number of slender forms was also seen at the 14th day for the clone Col-C14 (48%).
Statistical analysis
Comparative analysis between the percentages of slender and broad forms between the parental strain and its clones in the 14th an 20th days of infection revealed significant differences by the X2 chi-square nonparametric test, as follows:
Slenders forms
Significant differences were detected between the Col-P strain and the clones Col-C1, Col-C8, Col-C10, Col-C13 and Col-C14 (P-values varied from P = 0.00001 a P = 0.001). No significant differences were detected between the Col-P strain and the clone Col-C15 (P = 0.9).
Broad forms
Significant differences were observed detected Col-P strain and its clones Col-C5, Col-C8, Col-C10, Col-C14 and Col-C15 (P values varied from P = 0.0000001–0.003). No significant differences were detected between Col-P strain and the clones Col-Col-C1 (P = 0.3) and Col-C13 (P = 0.1).
Cumulative mortality
Cumulative mortality varied between the several clones, from 40 to 100%. Statistical analysis of the cumulative mortality (Fischer exact Test) demonstrated significant difference between the Colombian parental strain (Col-P) and the clones Col–C5, Col-C8, Col-C10, Col-C13 and Col-C14 (P = 0.00005–0.01). No significant difference was detected between Col-P and the clones Col-C1 (P = 0.7) and Col-C15 (P = 0.2).
Virulence
The virulence of the several clones and parental strain varied considering the parameters cumulative mortality, survival time and levels of parasitemia.
Col-P (parental strain), clones Col-C13 and Col-C14 showed higher virulence than the clones Col-C8, Col-C10 and Col-C15. Clones Col-C1 and Col-C5, isolated in the 30th day of infection, also differed in virulence, as previously demonstrated (Camandaroba et al. 2001). Clone ColC–C1 showed high virulence, determining high mortality rate (100%) of the infected mice, high parasitemia and presence of slender forms in the 14th day of infection (Figure 1).
Isoenzymic profiles
The patterns of isoenzymes ALAT, ASAT, PGM and GPI reproduce the same profiles as seen previously for the Colombian strain and the clones isolated in the 30th day, with the profile of Zymodeme 1 (Camandaroba et al. 2001).
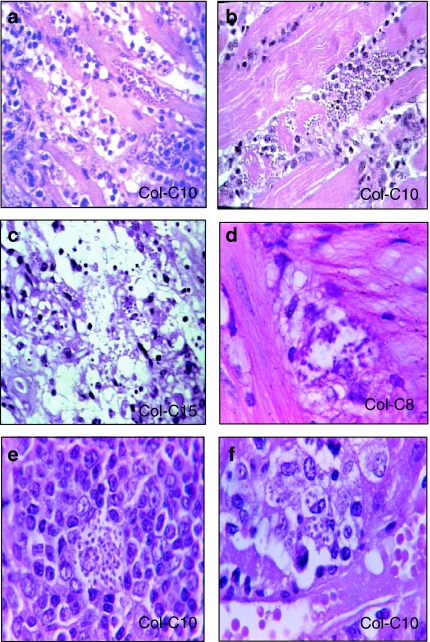
Histopathological lesions (Figures 3 and 4)
Figure 3.
Histotropism of clones of the Colombian strain. (a) Clone Col-C10 – section of the heart with intracellular parasites, necrotic myocytes and mononuclear cells infiltrates in substitution of destroyed cardiac cells, H&E ×400. (b) Clone Col C-10 – skeletal muscle showing parasitized myocyte, cellular disintegration and mononuclear cells infiltration, H&E ×400. (c) Clone Col-C15 – Adipocytes (arrows) in the pericardiac connective tissue, containing amastigotes of Trypanosoma cruzi, H&E ×400. (d) Clone Col-C8 – neuronal cells of the para-sympathetic ganglium of the Auerbach plexus in the intestinal wall containing amastigote forms of T. cruzi. H&E ×1000. (e) Clone Col-C10 – section of the spleen showing vacuolized macrophage in the lymphoid follicle, containing amastigote forms. H&E ×400. (f) Clone Col-C10 – macrophage in the hepatic sinusoid (Kupffer cell), containing amastigotes of T. cruzi, H&E ×1000.
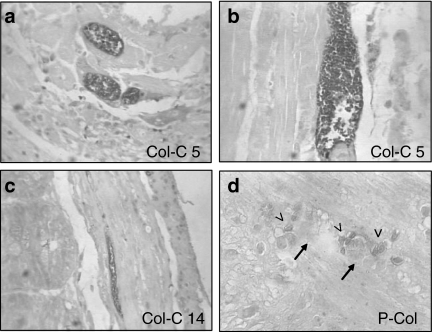
Figure 4.
Immuno-histochemical labelling of Trypanosoma cruzi amastigotes by peroxidase: (a) Section of the heart with mononuclear cells infiltration and the presence of intracellular amastigote forms of T. cruzi. (×400). (b) Skeletal muscle showing a large nest of amastigote forms immunolabelled with anti-T. cruzi antibodies. (×400). (c) Clone Col-C5 – section of the intestine, showing amastigotes of T. cruzi inside the smooth muscle cells of the intestinal wall. (×100). (d) Clone P-Col (parental strain) – presence of amastigotes (arrow head) in the Auerbach plexus (arrows) of the intestinal wall, ×400.
Parental strain and clones
The progressive histopathological study in mice inoculated either with the parental Colombian strain or with the clones isolated from early infection (10th day) showed variable aspects, considering the intensity of the parasitism and degree of inflammation in the myocardium and skeletal muscle. These lesions were generally mild or absent at the 10th and 14th day of infection, increasing in severity from the 20th to 25th day and becoming very intense in the 30th day postinfection. The evolution of these lesions was similar to that observed in mice infected with the Colombian parental strain. An increasing severity of the lesions in the myocardium and skeletal muscles was observed with the presence of nests of amastigotes in the myocytes and inflammatory lesions, corresponding to the rupture of the parasitized cells. An intense diffuse infiltration of the myocardium and skeletal muscle with mononuclear cells was seen in the advanced phase of infection (20th to 30th day) (Figure 3a,b). Para-vertebral sympathetic ganglia and para-sympathetic peri-cardiac ganglia were involved by the inflammatory infiltrate present in the muscles and adipose tissue, but no parasites were found in these structures.
Histotropism
Table 1 summarizes the presence of intracellular amastigotes in several tissues of mice infected either with the parental strain or the clones isolated in the 30th days of infection (Col-C1 and Col-C5) or in the 10th day of infection. Parasites were seen in sections stained with haematoxylin and eosin (Figure 3) and in sections submitted to histochemistry for the identification of T. cruzi forms (Figure 4). Parasites were identified in the skeletal muscle and myocardium (Figures 3a,b and 4a,b); smooth muscle of the intestinal wall (Figure 4c); macrophages in the spleen and liver (Figure 3e,f); in adipocytes (Figure 3c) and in satellite and neuronal cells of nervous ganglia of the Auerbach plexus (Figures 3d and 4d).
Table 1.
Presence of intracellular amastigotes in different tissues of mice infected with the Colombian strain of Trypanosoma cruzi and its clones
| Muscle | Intestines | |||||
|---|---|---|---|---|---|---|
| Colombian strain and clones | Heart | Skeletal | Spleen and Liver macrophages | Colon* | Rectum* | Auerbach Plexus** |
| Parental strain | +/+++ | +/+++ | + | + | + | + |
| Cl-Col-C1 | +/+++ | +/++ | + | + | + | + |
| Cl-Col-C5 | +/+++ | +/+++ | + | + | + | + |
| Cl-Col-C8 | +/++ | + | + | + | – | – |
| Cl-Col-C10 | +/+++ | +/+++ | + | + | + | – |
| Cl-Col-C13 | +/++ | + | + | – | – | – |
| Cl-Col-C14 | +/+++ | +/+++ | + | –/+ | –/+ | – |
| Cl-Col-C15 | +/+++ | +/+++ | + | +/++ | +/++ | + |
(+) Mild degree; (++) Moderate degree; (+++) Intense degree of parasitism in different tissues as found in two 5-µm sections of each examined organ stained with H&E and/or by peroxidase reaction with anti-T. cruzi-purified antibody.
(–) Parasites were not detected by examination of 5-µm-thick sections.
Parasites in the smooth muscle cells.
Parasites in the neuronal or satellite cells.
Discussion
Classically, macrophages are the first barrier to T. cruzi invasion after infection, either in man or in the experimental animals. Parasites actively phagocytosed can be destroyed or can develop within the phagocyte cytoplasm. This is followed by dissemination of parasites in the vertebrate organism and their intracellular multiplication in different tissues. The histotropism is a hallmark of the different strains of T. cruzi, and this means a predominance of parasite multiplication into one tissue but not excluding its multiplication in other tissues. Macrophages, skeletal and cardiac muscles, or intestinal smooth muscles are the preferential sites of parasite multiplication. Strains of T. cruzi, which were biologically and biochemically characterized and classified into three biodemes (Andrade & Magalhães 1997), showed different histotropism during the acute infection in mice.
The predominant tropism of one biodeme is preserved, independently of the mouse strain used as observed with the Colombian strain when inoculated into six different inbred mouse strains: DBA/1, C3H, B/10, BALB/c, AKR and A/J, always presenting a defined skeletal muscle tropism (Andrade et al. 1985). Concomitantly, parasites are present in low number, in several other tissues, as the spleen, liver, neuronal ganglia, smooth muscles and adipose tissue. In the present study, the same tropism of the parental strain has been observed with the isolated clones.
The clones isolated in the 10th day of infection varied in virulence, evaluated by the mortality indices, survival time and parasitemic levels. Considering these parameters, the parental strain as well as clones C-13 and C-14 were the most virulent, with high mortality and high levels of parasitemia. Predominance of slender forms in the early phase (14th days) indicates a rapid multiplication as has been seen with the parental strain and the clone C-14. This coincided with the presence of macrophagotropism in the spleen and liver. The parameters of infectivity, pathological lesions and histotropism demonstrate the similarity between the clones and respective parental strain. Previous studies from our laboratory have demonstrated the clonal homogeneity of the 21 SF strain, biodeme Type II both at phenotypic characterization (Campos & Andrade 1996) and schizodeme analysis (Campos et al. 1999). The schizodemes patterns of several clones isolated from the Colombian strain of T. cruzi confirmed the homogeneity of the clones and suggest the predominance of a ‘principal clone’ in this strain.
These studies indicated that even considering the strains as discrete type unities (DTUs), as defined by Tibayrenc & Ayala (2002), they presented clear tropism for different types of cells.
In the present investigation, we could demonstrate that clones isolated from the Colombian strain reproduced the pathological involvement of the heart and skeletal muscles as well as the histotropism for the cells of several tissues, as seen with the parental strain, either when isolated from an early phase of infection or at the peak of parasitemia. These data are not in accordance with the theory of the ‘clonal histotropic model’ that has been described by Macedo & Pena (1998), followed by others (Andrade et al. 1999, 2002). In the pioneering studies by Vago et al. (1996a, 2000), they suggested that among the multiclonal population infecting an individual, there could be clones with specific tropism to different tissues. In the study of necropsy specimens, from T. cruzi chronically infected individuals, Vago et al. (1996a, b, 2000) have shown the possibility to directly identify in the heart and digestive tract tissues the presence of genetically different parasites by the technique of LSSP-PCR. However, the possibility that these patients had been infected with different strains has not been discharged by the authors. Franco et al. (2003) by inoculating rats with a mixture of two different T. cruzi populations monoclonal JG strain and the CL-Brener clone have shown, at the end of acute phase, the presence in the heart of only JG strain kDNA, while skeletal muscle and rectum exhibited only CL-Brener kDNA. In the chronic infection, JG appeared in the heart and other organs previously parasitized by CL-Brener strain. These results seems to confirm that different strains or clones can present different tropism. However, the presence of the JG monoclonal strain in several tissues is a demonstration that the same clone can parasitize different types of cells, as shown in the present study.
It is known that re-infection can occur in patients living in endemic areas; experimentally, parasites of double infection have been re-isolated from mice infected with different strains of T. cruzi (Andrade et al. 1968; Deane et al. 1984). In endemic areas, domiciliary invasion by sylvatic triatominae is responsible for the presence of infections with different strains, as it has been shown in patients from Montalvania, MG (Luquetti et al. 1986; Andrade et al. 1992).
In the present study, we have demonstrated, by direct identification of parasites in the tissues, that clones derived from one single trypomastigote, isolated by micromanipulation and inoculated into mice, determine the parasitism of the cells of different organs and tissues and disclosed the same distribution and histotropism as the parental strains. These findings suggest that the tissue distribution of one clone, similarly to the Colombian parental strain, is ubiquitous and not restrictive.
References
- Andrade SG. Caracterização de cepas do Trypanosoma cruzi isoladas no Reconcavo Bahiano. Rev. Pat. Trop. 1974;3:65–121. [Google Scholar]
- Andrade SG. Trypanosoma cruzi: clonal structure of parasite strains and the importance of principal clones. Mem. Inst. Oswaldo Cruz. 1999;94:185–187. doi: 10.1590/s0074-02761999000700026. [DOI] [PubMed] [Google Scholar]
- Andrade V, Barral-Netto M, Andrade SG. Patterns of resistance of inbred mice to Trypanosoma cruzi are determined by parasite strain. Braz. J. Med. Biol. Res. 1985;18:499–506. [PubMed] [Google Scholar]
- Andrade V, Brodskyn C, Andrade SG. Correlation between isoenzyme patterns and biological bechavior of different strain of Trypanosoma cruzi. Trans. Roy. Soc. Trop. Med. Hyg. 1983;77:796–799. doi: 10.1016/0035-9203(83)90292-4. [DOI] [PubMed] [Google Scholar]
- Andrade SG, Figueira RM, Andrade ZA. Influência de infecções repetidas no quadro histopatológico de doença de Chagas experimental. Gaz. Med. Bahia. 1968;68:115–123. [Google Scholar]
- Andrade LO, Machado CRS, Chiari E, Pena SDJ, Macedo AM. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol. Bioch. Parasitol. 1999;100:163–172. doi: 10.1016/s0166-6851(99)90035-x. [DOI] [PubMed] [Google Scholar]
- Andrade LO, Machado CRS, Chiari E, Pena SDJ, Macedo AM. Trypanosoma cruzi: role of host genetic background in the differential tissue distribution of parasite clonal populations. Exp. Parasitol. 2002;100:269–275. doi: 10.1016/s0014-4894(02)00024-3. [DOI] [PubMed] [Google Scholar]
- Andrade SG, Magalhães JB. Biodemes and zymodemes of T. cruzi strains: correlations with clinical data and experimental pathology. Rev. Soc. Bras. Med. Trop. 1997;30:27–35. doi: 10.1590/s0037-86821997000100006. [DOI] [PubMed] [Google Scholar]
- Andrade SG, Rassi A, Magalhães JB, Ferriolli Filho F, Luquetti AO. Specific chemotherapy of Chagas disease: a comparison between the response in patients and experimental animals inoculated with the same strain. Trans. Roy. Soc. Trop. Med. Hyg. 1992;86:624–626. doi: 10.1016/0035-9203(92)90156-7. [DOI] [PubMed] [Google Scholar]
- Camandaroba ELP, Campos RF, Magalhães JB, Andrade SG. Clonal structure of Trypanosoma cruzi Colombian strain (biodeme Type III): biological, isoenzymic and histopathological analysis of seven isolated clones. Rev. Soc. Bras. Med. Trop. 2001;34:151–157. doi: 10.1590/s0037-86822001000200001. [DOI] [PubMed] [Google Scholar]
- Camandaroba ELP, Reis EAG, Gonçalves MS, Reis MG, Andrade SG. Trypanosoma cruzi: susceptibility to chemotherapy with benznidazole of clones isolated from the highly resistant Colombian strain. Rev. Soc. Bras. Med. Trop. 2003;36:201–209. doi: 10.1590/s0037-86822003000200002. [DOI] [PubMed] [Google Scholar]
- Campos RMF, Andrade SG. Characterization of subpopulations (Clones and Subclones) of the 21 SF strain of Trypanosoma cruzi after long lasting maintenance in the laboratory. Mem. Inst. Oswaldo Cruz. 1996;91(3):795–800. [PubMed] [Google Scholar]
- Campos RMF, Gonçalves MS, Reis EAG, Reis MG, Andrade SG. Comparative analysis by polymerase chain reaction amplified minicircles of kinetoplast DNA of a stable strain of Trypanosoma cruzi from São Felipe, Bahia, its clones and subclones: possibility of predominance of a principal clone in this area. Mem. Inst. Oswaldo Cruz. 1999;94:23–29. doi: 10.1590/s0074-02761999000100009. [DOI] [PubMed] [Google Scholar]
- Deane MP, Sousa MA, Pereira NM, Gonçalves AM, Momem H, Morel C. Trypanosoma cruzi: inoculation schedules and re-isolation methods select individual from doubly infected mice, as demonstrated by schizodeme and zymodeme analysis. J. Protozool. 1984;31(2):276–280. doi: 10.1111/j.1550-7408.1984.tb02960.x. [DOI] [PubMed] [Google Scholar]
- Dvorak JA. Single cell isolates of Trypanosoma cruzi: how and why? Rev. Soc. Med. Trop. 1985;18:29–38. [Google Scholar]
- Dvorak JA, Hartman DL, Miles MA. Trypanosoma cruzi: correlation of growth kinetics to zymodeme type in clones derived from various sources. J. Protozool. 1980;27:472–474. [Google Scholar]
- Engel JC, Dvorak JA, Segura EL, Crane MJ. Trypanosoma cruzi: biological characterization of 19 clones derived from two chagasic patients. I. Growth kinetics in liquid medium. J. Protozool. 1982;29:555–560. doi: 10.1111/j.1550-7408.1982.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Franco DJ, Vago AR, Chiari E, Meira FC, Galvão LM, Machado CR. Trypanosoma cruzi: mixture of two populations can modify virulence and tissue tropism in rat. Exp. Parasitol. 2003;104:54–61. doi: 10.1016/s0014-4894(03)00119-x. [DOI] [PubMed] [Google Scholar]
- Goldberg SS, Silva PAA. Enzyme variation among clones of Trypanosoma cruzi. J. Protozool. 1983;69:91–96. [PubMed] [Google Scholar]
- Gomes ML, Romanha AJ, Gonçalves AM, Chiari E. Stability of isoenzyme and kinetoplast DNA (k-DNA) patterns in sucessively cloned Trypanosoma cruzi populations. Mem. Inst. Oswaldo Cruz. 1991;86:379–385. doi: 10.1590/s0074-02761991000400001. [DOI] [PubMed] [Google Scholar]
- Lima MT, Jansen AM, Rondinelli E, Gattass CR. Trypanosoma cruzi: properties of a clone isolated from CL strain. Parasitol. Res. 1990;77:77–81. doi: 10.1007/BF00934390. [DOI] [PubMed] [Google Scholar]
- Luquetti AO, Miles MA, Rassi A, et al. Trypanosoma cruzi: zymodemes associated with acute and chronic Chagas disease in central Brazil. Trans. Roy. Soc. Trop. Med. Hyg. 1986;80:462–470. doi: 10.1016/0035-9203(86)90347-0. [DOI] [PubMed] [Google Scholar]
- Macedo AM, Pena SDJ. Genetic variability of Trypanosoma cruzi: implications for the pathogenesis of Chagas disease. Parasitol. Today. 1998;14(3):119–124. doi: 10.1016/s0169-4758(97)01179-4. [DOI] [PubMed] [Google Scholar]
- Marques AS, Chiari E. Caracterização biológica de clones das cepas Y, CL e MR de Trypanosoma cruzi em camundongos C3H isogênicos. Mem. Inst. Oswaldo Cruz. 1988;83:175–181. doi: 10.1590/s0074-02761988000200005. [DOI] [PubMed] [Google Scholar]
- Miles MA, Lanham SM, Souza AA, Povoa M. Further enzymic characters of Trypanosoma cruzi and their evaluation for strain identification. Trans. Rov Soc. Trop Med. Hyg. 1980;74:221–237. doi: 10.1016/0035-9203(80)90251-5. [DOI] [PubMed] [Google Scholar]
- Momem H. Taxonomy of Trypanosoma cruzi: a commentary on characterization and Nomenclature. Mem. Inst. Oswaldo Cruz. 1999;94:181–184. doi: 10.1590/s0074-02761999000700025. [DOI] [PubMed] [Google Scholar]
- Morel C, Chiari E, Plessmann Camargo E, Mattei DM, Romanha AJ, Simpson L. Strains and clones of Trypanosoma cruzi can be characterized by pattern of restriction endonuclease products of kinetoplast DNA minicircles. Proc. Natl. Acad. Sci. USA. 1980;77:6810–6814. doi: 10.1073/pnas.77.11.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza MM, Andrade SG, Barbosa AA, Santos RTM, Alves VAF, Andrade ZA. Trypanosoma cruzi strains and autonomic nervous system Pathology in experimental Chagas disease. Mem. Inst. Oswaldo Cruz. 1996;91(2):217–224. doi: 10.1590/s0074-02761996000200018. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M, Ayala FJ. The clonal theory of parasitic protozoa: 12 years on. Trends Parasitol. 2002;18:405–410. doi: 10.1016/s1471-4922(02)02357-7. [DOI] [PubMed] [Google Scholar]
- Vago AR, Andrade LO, Leite AA, et al. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease. Am. J. Pathol. 2000;156:1805–1809. doi: 10.1016/s0002-9440(10)65052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago AR, Macedo AM, Adad SJ, Reis DD, Oliveira RC. PCR detection of Trypanosoma cruzi DNA in oesophageal tissues of patients with chronic digestive Chagas'disease. Lancet. 1996a;348:891–892. doi: 10.1016/S0140-6736(05)64761-7. [DOI] [PubMed] [Google Scholar]
- Vago AR, Macedo AM, Oliveira RP, et al. Kinetoplast DNA signatures of Trypanosoma cruzi strains obtained directly from infected tissues. Am. J. Pathol. 1996b;149:2153–2159. [PMC free article] [PubMed] [Google Scholar]