Abstract
Although dengue virus (DV) enters through skin while mosquitoes feed, early contacts remain unexplored regarding the cutaneous viral fate and in situ immune responses. We addressed this by exposing healthy, non-cadaveric, freshly obtained human skin explants to a human DV2 isolate. We demonstrated negative-strand DV-RNA and non-structural protein-1, both suggestive of viral replication in skin. Although control, mock-infected and DV-infected explants showed less (MHC-CII+/CD1a+/Langerin+) Langerhans cells, deranged morphology and decreased frequency were more apparent in DV-infected explants. Whereas DV+ cells were infrequent in epidermis and completely absent in dermis, some areas of basal epidermis were clearly DV+, presumably keratinocytes, cells where TUNEL positivity revealed apoptosis. Unlike fresh, control and mock-infected skin, DV-infected explants expressed CD80 and CD83, indicative of dendritic cell (DC) activation and maturation, respectively. However, sequential sections indicated that these cells were not DV+, suggesting that activated/mature DCs capable of priming T cells, probably, were not infected. Alternatively, the occasionally infected epidermal DC might not have reached maturation. Interestingly, skin DV infection apparently uncouples the DC activation/maturation process from another crucial DC function, the subsequent migration into dermis. This was suggested, because upon cutaneous DV infection, the few emerging CD83+ (mature) DCs remained within the outer epidermis, while no dermal CD83+ DCs were observed. These paradoxical effects might represent unknown DV subversion strategies. This approach is relatively easy, quick (results in 48 h), economical for developing countries where dengue is re-emerging and advantageous to evaluate in situ viral biology, immunity and immunopathology and potential antiviral strategies.
Keywords: dengue virus, human skin, in situ infection, Langerhans cells
Dengue virus (DV) infections are serious causes of morbidity and mortality worldwide, and adaptive (secondary) immune response appears to influence the severity of this arboviral disease (Morens 1994; Halstead & O'Rourke 1977; Rigau-Perez et al. 1998). There are an estimated 50–100 million cases of dengue fever and 250,000-500,000 cases of dengue haemorrhagic fever (DHF) annually in the world (WHO 1997). The dengue shock syndrome (DSS), the most severe and potentially fatal form of the disease, especially in developing countries, is less frequent. In the model proposed here, we have chosen to examine DV2, because it is the prevalent serotype (Gomez-Dantes et al. 2004). The high seroprevalence together with the co-circulation of multiple serotypes indicates that Mexico could be in risk of an important DHF outbreak (Gomez-Dantes et al. 2004). The exact pathogenic mechanisms following DV infection are not well understood, especially in the severe forms of the infection (DHF and DSS). The immune response to DV seems at least partially involved in the process (Halstead 1988; Avirutnan et al. 1998; Diamond et al. 2000). Several theories have been advanced including facilitation of DV infection by non-neutralizing antibodies (Halstead & O'Rourke 1977; Halstead et al. 1977; Morens 1994), the potential differential virulence of each infecting serotype (Leitmeyer et al. 1999; Kawaguchi et al. 2003), CD4-CD8 T-cell cross-reactivity and host factors such as a particular, but yet undefined susceptibility (Klenerman & Zinkernagel 1998; Mongkolsapaya et al. 2003). While there is an excellent research on dengue about the molecular structure and epidemiology of this virus, the basic mechanisms of the immunopathology still remain obscure (Haywood 1994; Kuhn et al. 2002). For instance, until very recently (Johnston et al. 2000; Wu et al. 2000), it was unknown whether DV was able to replicate in cutaneous cells. Likewise, there are two other features largely unexplored regarding DV infection: (i) the very early stages of the infection, because, by necessity, patients are examined when disease is almost over, when the patients are under recovery or when they have entered into DSS and (ii) which type of responses, if any, occur cutaneously, in situ, following DV inoculation when mosquitoes feed.
We wanted to develop an in situ approach that would enable us to study the early immune pathology of the cellular events regarding the infectious process and the first reactions of the peripheral immune system, the dendritic cells (DCs). DCs constitute a system of highly dynamic sentinel cells, whose most studied element in peripheral non-lymphoid tissues is the epidermal Langerhans cells (LCs). It is now known that LCs perform multiple tasks including antigen capturing and processing, antigen ferrying to regional lymphoid tissues, and ultimately, cognate antigen presentation to naïve lymphocytes, once in secondary lymphoid organs (Flores-Romo 2001). Under the influence of a variety of stimuli such as cytokines, antigens or microbial products, LCs become activated, start to migrate into the dermis and concomitantly to up-regulate antigen-presenting molecules and to express co-stimulatory and maturation markers (Cumberbatch & Kimber 1992; Cumberbatch et al. 1997). Nevertheless, despite that LCs as sensors of the external antigenic world are the most exposed cells of the immune system during mosquito feeding, and the initial virus inoculation through skin, the interactions of these cells with DV and the outcome of such potential interplay regarding both the virus and the LC have received little attention in situ (Wu et al. 2000).
We attempted to address some of these issues by establishing a model with fresh, healthy, non-cadaveric human skin explants exposed to DV, to analyse whether local infection and viral replication are feasible experimentally, to assess the most early phases of the initiation of the immune responses in situ and the immediate local repercussions of viral inoculation upon local antigen-presenting cells (APCs), particularly on the DCs, the sentinel posts of the immune system in the skin.
Material and methods
Skin samples
Freshly prepared, non-cadaveric healthy human skin was obtained from five healthy women undergoing plastic surgery. Donation of samples was approved both by the patients and by the ethical committee of local hospitals. Skin obtained in sterile conditions was immediately placed in sterile vials containing endotoxin-free sterile saline solution and maintained in ice until use in the laboratory, which usually occurred within the first 3 h. Once, in the laboratory and under sterile conditions, skin was cut into pieces of approximately 1.0 cm2, excess of underlying fat was carefully removed and each piece was placed (epidermis side up) into individual wells in a 12 wells Costar culture plate (Costar corning, NY, USA). Results shown represent five experiments performed with the skin samples obtained from five healthy women undergoing plastic surgery. Unless otherwise indicated, pictures are also representative of the five different samples examined.
Dengue virus isolate and titration
DV was obtained and expanded from a blood sample taken from a patient suffering classical dengue. Serotyping of this new isolate as DV2 was performed as routinely done in the laboratories of the Institute for Epidemiological Diagnosis and References (INDRE), the official institution of Mexico's National Health System that diagnoses and typifies DV. To expand this new clinical isolate, the usual approach of DV inoculation into mice was used (Gould & Clegg 1991). Virus was then titrated by the standard plaque-forming assay using the BHK-21 cells (Talavera et al. 2004). Briefly, a 10-fold serial dilution of the virus was added to BHK-21 monolayers cultured in 24-well plates at 2.5 × 105 cells/ml, and these were incubated at 37 °C for 4 h. After this time, 0.5 ml minimum essential medium (MEM) containing 10% foetal bovine serum (FBS) and 3% (w/v) carboximethylcellulose was added to each well. After 5 days of culture at 37 °C, plaques were visualized by staining with naphtol blue black. Virus titres are given as plaque-forming units (pfu)/ml. Then, the capacity of this DV serotype to infect another classical, but very different and very susceptible target cell, the C6/36 insect cell, was also tested. Insect C6/36 mosquito cells from Aedes albopictus were kindly provided by Fernando Medina-Ramirez, Laboratory of Virology, CINVESTAV-IPN. The ability of this new viral isolate to infect these target insect cells was assessed both by the cytopathic effects and by identifying the negative-strand DV-RNA, indicative that viral replication has actively occurred in these cells (Lanciotti et al. 1992; Raengsakulrach et al. 2002). Once the infectious and cytopathic abilities of this viral isolate were verified in a very different susceptible target cell, we proceeded to inoculate the human skin explants.
Skin explants inoculation and culture conditions
DV infection of skin explants was attained by placing different multiplicity of infection (this is calculated by dividing the pfu between the number of cells to infect) in 30 µl of culture medium onto the skin sample to be tested, which was placed dermis side down. Then, without piercing the skin, we used a very fine (30-gauge) insulin syringe needle (HSW, Tuttlingen, Germany) to gently scarify the surface to spread the DV inoculum over the skin explants. Several control samples were included, such as untreated, freshly obtained skin, skin cultured in medium only and skin pieces which received exactly the same treatment but with samples obtained from uninfected mice (mock-infected skin). In the first 2 h of viral adsorption when the inoculum is allowed to interact only with the upper (epidermal) side, each skin portion is placed in a 24-well plate, dermis side down. Only this 2-h step is performed without culture medium, thus allowing us to restrict the inoculation onto the top, epidermal side. However, during these 2 h and also in all the subsequent culture periods, explants are always maintained in an incubator with a humid atmosphere and 5% CO2. In the second part of the process after this 2-h period, samples are thoroughly washed with sterile Hank's solution to remove any excess of virus load and then cultured in 0.5 ml of RPMI with 10% FBS, 100 U penicillin and 100 µg of streptomycin/ml of culture medium per well. Special care was taken to avoid overflowing the samples with culture medium. From this step onwards, all skin samples followed the same culture procedures for 48, 72 and 120 h.
RNA isolation and RT-PCR analysis
As an attempt to evaluate the viral replication, we used the protocol described by Lanciotti et al. (1992) to detect the intermediate negative-strand viral RNA, because DV has RNA of positive polarity. To this end, after the culture period of skin explants was finished, skin samples were immediately placed in 1.0 ml of TRIzol reagent (Invitrogen, Paisley, UK). RNA was purified according to the manufacturer's protocol, and RT-PCR was performed using specific primers for the negative-strand viral RNA, according to previous reports (Lanciotti et al. 1992).
In situ immunolabelling for cutaneous DCs, DV and non-structural protein-1
After the culture period was over, skin explants were removed, carefully washed with culture medium and two basic procedures followed: (i) A small area was vertically cut from each single skin sample, immediately placed in optimum cutting temperature (OCT) compound (Leica, Nussloch Germany), frozen and kept in liquid nitrogen until immunolabelling procedures took place and (ii) The larger skin portion was immediately incubated in an enzymatic cocktail containing collagenase type I (Sigma, St. Louis, MO, USA) and dispase (Gibco, Carlsbad, CA, USA) for 2 h at 37 °C. After this, epidermal sheets were carefully separated by gentle traction with fine forceps, stretched in sterile PBS-BSA and immediately fixed in cold (–20 °C) acetone for 20 min. Thorough washings followed using PBS-BSA before antibody labelling. Conventional 5.0 µm skin sections from frozen tissue were incubated overnight at 4 °C with primary antibodies, used at optimal dilutions previously determined for each one (Table 1). Primary antibodies and dilutions used were the following: CD1a 1:100 (Dako, Glostrup, Denmark), MHC-CII (DR) 1:100, CD80 1:40 and CD83 1:50 all purchased from Pharmingen (San Diego, CA, USA). Anti-human Langerin was obtained from Novocastra (Benton Lane, UK) and used at 1:100. After three washes, peroxidase-linked secondary sheep anti-mouse antibodies (Amersham Biosciences, Buckinghamshire, UK) were added at a dilution 1:250 for 1 h at room temperature followed by extensive washing. Developing of bound antibodies was achieved using 3-3'-Diaminobenzidine (DAB) for brown colour (Gibco, Carlsbad, CA, USA) or the kit SG (Vector, Burlingame, CA, USA) for blue dark colour. The mouse antibody specific for DV recognizes the structural protein E (envelope) of DV, was purchased from the P. Kouri Tropical Medicine Institute (Havana, Cuba) and used at a dilution 1:100. Our group recently developed a murine monoclonal antibody to recombinant non-structural protein-1 (rNS1), an essential component of the viral replicase. This antibody was used at a dilution 1:5. Counterstaining in this case was done with Nuclear Fast red. In the case of epidermal sheets, these were cut into small squared portions of approximately 5 mm to use at least three of these pieces for each different antibody labelling. Primary antibodies used and dilutions (Table 1) were the same as for skin sections, only that each single piece of epidermal sheet was incubated in 50 µl of the corresponding antibody dilution and then washed ×3 in 300 µl of PBS-BSA. Colour reaction development for epidermal sheets was the same as for skin sections. Once colour development was stopped for both procedures, samples were placed in mounting media Immunomount (Shandon, Pittsburgh, PA, USA), and after carefully stretching the epidermal sheets portions to make them as flat as possible, they were coverslipped to be assessed in a Carl Zeizz microscope (Carl Zeiss, Oberkochen, Germany). Positive cells for any given marker were counted in 10-20 different fields, using a calibrated grid and at least three portions of epidermal sheet from each subject. Cell quantification per tissue area was performed blinded using coded slides with the different tissue samples. Finally, cell counts were reported as positive cells per square mm of epidermal tissue.
Table 1. Summary of antibodies used in the present study.
| Antibody | Source | Cat. Number | Dilution used |
|---|---|---|---|
| CD1a | Dako, Glostrup, Denmark | M0721 | 1:100 |
| MHC-Cll (DR) | Dako, Glostrup, Denmark | M0704 | 1:100 |
| CD80 | Pharmingen, San Diego, CA, USA | 33514X | 1:50 |
| CD83 | Pharmingen, San Diego, CA, USA | 556910 | 1:40 |
| Langerin | Novocastra, Benton Lane, UK | VPL 552 | 1:100 |
| Anti-mouse Ig-POX | Amersham Bioscience, Buckinghamshire, UK | NA931 | 1:250 |
| Anti-DV2 | P. Kouri Tropical Medicine Institute, Havana, Cuba | 1:100 | |
| Anti-rNS1 | Laboratory of Virology, Department of Biomedicine, CINVESTAV-IPN | 1:50 |
Apoptosis by TUNEL technique
To ascertain the apoptotic cell death in the model of skin explants, we used the Deadend fluorometric TUNEL system (Promega, Madison, WI, USA) in the cultured samples to evaluate the nuclear DNA fragmentations. The various forms of cultured explants were taken once the culture period was finished, washed carefully in sterile PBS-BSA and frozen in OCT compound (Leica, Nussloch, Germany). Negative controls included omission of Dnase, whereas positive controls consisted of sections of the same samples subjected to Dnase treatment. TUNEL positivity is revealed by green fluorescence, and counterstaining was done using propidium iodide which provides an intense red staining.
Results
Cytopathic effects of DV2 isolate on target insect C6/36 cells
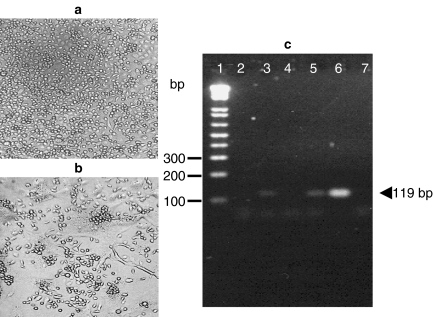
Before testing the potential effects of this DV isolate in human skin explants, we wanted to assess the infectivity and cytopathic activities of this new DV2 clinical isolate. Thus, we tested this DV sample in a classical target cell, the C6/36 mosquito cell line, which is known for its high susceptibility to both the infection and the cytopathic properties of this virus. As shown in Figure 1b, the DV2 cytopathic effects were clearly seen at 48 h of culture, compared with the control cultures of mosquito cells (Figure 1a). Likewise, detection of the negative-strand intermediate viral RNA was readily observed in these cell extracts (Figure 1c). Both the pathogenic activities observed in the mosquito cells, as well as the putative viral replication (intermediate RNA), suggested to us that this new DV isolate was appropriate to be tested in our system of cultured whole skin explants.
Figure 1.
Cytopathic effects of the new dengue virus 2 (DV2) clinical isolate on target insect cells and RT-PCR from conventional target mosquito cells and from cultured explants of human skin. The classical mosquito cell-line C6/36 (Aedes albopictus) was used to test whether this new viral isolate from a patient would infect a conventional target cell. 107 C6/36 cells were infected using a multiplicity of infection of 0.1 PFU/cell in tissue-culture flasks of 25 cm2 (Corning, NY, USA) and humid atmosphere with 5% CO2 at 37 °C for 2 days. DV2 was added, since the beginning of culture, and 24 h later, the typical cytopathic effects were observed (b), compared with control non-infected cultures (a). To detect the negative-strand DV-RNA indicative of active viral replication, RT-PCR was done with RNA obtained from control-cultured non-inoculated and DV-inoculated skin explants, as well as conventional target (C6/36) mosquito cells. Lane 1: molecular weight markers, lane 2: cultured non-inoculated skin explants, lane 3: DV-inoculated explants cultured for 48 h, lane 4: cultured non-inoculated explants (72 h), lane 5: DV-inoculated explants cultured for 72 h, lane 6: mosquito cells infected with DV2 isolate and lane 7: PCR negative control. Arrowhead indicates the negative-strand viral amplicon (119 bp size). Results are representative of five different experiments from skin samples obtained from five individuals.
DV2 isolate apparently infects and replicates efficiently in healthy human skin explants ex vivo
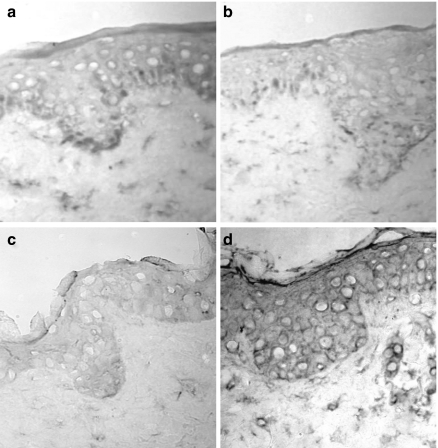
First, we wanted to know whether healthy non-cadaveric human skin explants in culture would provide a suitable tissue microenvironment as a model to achieve experimental DV2 infection ex vivo. By means of specific primers (Lanciotti et al. 1992) and performing RT-PCR, we demonstrated first the appearance of negative-strand viral RNA (Figure 1c lanes 3 and 5, arrowhead) both at 48 and 72 h. This suggested that active viral replication has occurred in the settings of cultured whole skin explants. Furthermore, by using a murine monoclonal antibody to rNS1 recently developed in our group, we also found that, unlike freshly prepared, control-cultured or mock-infected skin, only DV-inoculated skin showed positivity for NS1. Both findings, the negative-strand viral RNA plus the presence of NS1 (shown in Figure 4), are highly suggestive of active viral replication in situ.
Figure 4.
Non-structural protein-1 (NS1) can be identified only in dengue virus (DV)-inoculated explants. Tissue sections obtained from the skin explants under different treatments were all incubated with a murine monoclonal antibody to recombinant NS1 from DV. Positivity was developed with peroxidase to obtain blue dark colour, while counterstaining was performed with nuclear fast red, providing a reddish staining. (a) illustrates sections from freshly prepared skin, (b) skin sections from controlcultured explants, (c) skin from mock-infected explants and (d) the blue dark labelling obtained in explants inoculated with DV. Pictures are representative of three different subjects assessed.
In situ effects of DV infection upon the local APC: the Langerhans cells
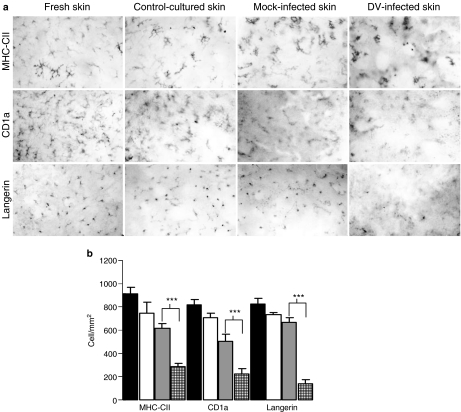
Epidermal sheets by providing an en face‘planar’ view represent perhaps the most appropriate tool to assess any particular spatial distribution of LC, quantification of their frequency per skin area, as well as to evaluate morphological changes even at the level of individual cells, for instance to follow the dendrites extension or retraction, etc. Figure 2a, far left panel (top to bottom pictures), shows the typical features of dendritic morphology and regular distribution of LC in fresh, non-infected, non-cultured skin when assessed with three different LC markers (MHC-CII, CD1a and Langerin). Figure 2a, middle left panel (top to bottom pictures) from skin explants cultured in medium without DV2, showed no overt changes, except perhaps for some cells with signs of activation, such as a rather increased MHC-CII expression and an accentuation of the dendritic morphology. Figure 2a, middle right panel (top to bottom pictures), illustrates the cells in mock-infected cultures, with similar features to the control-cultured explants. Figure 2a, far right panel (top to bottom pictures), shows a rather deranged distribution and morphology for LC in DV2-inoculated skin explants, especially for MHC-CII and Langerin. Almost the majority of the LC in DV2-infected explants seemed to have lost their typical dendritic appearance as that exhibited in fresh, untreated or control-cultured skin explants. Likewise, the typical regular pattern of the LCs-ordered spatial distribution looked disturbed in epidermal sheets of DV-infected explants. The decreased frequency of LC induced by DV infection was quantified by these three markers (CD1a, MHC-CII and Langerin) and is represented in Figure 2b.
Figure 2.
Effects of dengue virus 2 (DV2) infection upon epidermal dendritic Langerhans cells (LC). Monoclonal antibodies to MHC-CII (DR), CD1a and Langerin were used to assess epidermal LC. (a) Far left panel (top to bottom pictures) shows epidermal sheets from freshly obtained skin; middle left panel (top to bottom) represents non-inoculated cultured explants; middle right panel (top to bottom) represents mock-infected explants; far right panel (top to bottom) illustrates DV-inoculated skin explants. Pictures were taken at 5 days of culture and are representative examples of five different skin samples evaluated. (b) MHC-CII, CD1a and Langerin were used as markers to quantify LC frequency per area in each of these different experimental conditions. Closed bars indicate LC frequency in epidermal sheets from freshly (non-cultured, non-infected) obtained skin; open bars indicate LC in cultured non-infected skin; grey bars illustrate LC in mock-infected explants; squared bars indicate LC in DV-infected skin explants. Results are expressed as the mean ± SD of five different experiments from skin samples obtained from five individuals. Statistically significant differences are indicated by asterisks (***P < 0.001).
DV2+ cells can be identified in the basal layer of the epidermis of DV-inoculated explants
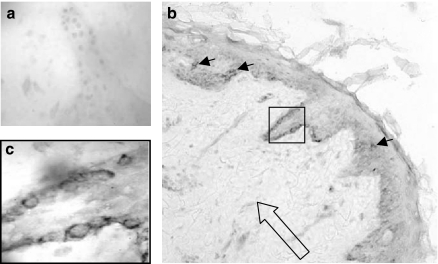
Upon DV inoculation, we looked for DV2+ cells in the skin explants cultured for 5 days. Initially, we experienced some difficulties to identify the uncommon DV+ DCs in epidermis, because they were rather scarce, but they were usually located in the suprabasal layer (Figure 3b, small arrows). Of note, DV+ cells in the dermis were completely absent (Figure 3b, large open arrow). Both findings were consistent in the skin samples from all five different subjects examined. However, in certain areas of the skin, DV+ cells were readily identified in basal cells of the epidermis (square in Figure 3b and magnification in Figure 3c). By virtue of the histological location, abundance and the morphology of these epidermal DV+ cells, it is highly likely that they are keratinocytes.
Figure 3.
Dengue virus 2 positive (DV2+) cells can be identified in the basal layer of the epidermis. Conventional sections of skin explants cultured for 5 days after DV2 inoculation clearly revealed DV positivity in many cells of the basal epidermis (b, square). Inset (c) shows the intense blue dark labelling in basal cells of epidermis, while very few DV+ cells were identified in the suprabasal layer (b, small arrows), presumably Langerhans cells. In contrast, no DV+ blue cells were observed in the dermis (b, large open arrow). Lack of staining with an isotype-matched control antibody is shown (a). Inset in (c) represents a magnification from (b) to illustrate the typical DV (dark blue) labelling representative of skin samples from five different individuals.
NS1 positivity in the epidermis of DV-inoculated explants
Because we recently developed a murine monoclonal antibody against rNS1 of DV, we used this reagent to evaluate our model of skin explants. Unlike the antibody to DV, NS1 positivity in epidermis was less restricted (Figure 4d), but this was not surprising, because this protein is secreted to the extracellular environment (Winkler et al. 1989). In contrast, freshly obtained control skin, control-cultured and mock-infected skin (Figure 4a–c) were devoid of any labelling for the NS1 DV protein.
Local effects of DV infection upon co-stimulatory/maturation markers on epidermal Langerhans cells
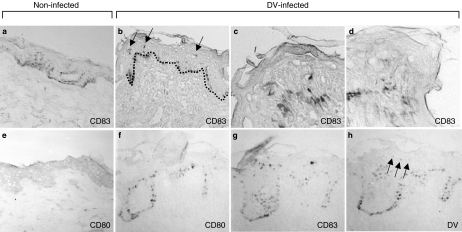
Because one of the earliest changes described for LC when they are somehow perturbed from their resting or ‘steady state’ conditions either by antigens, cytokines or by microbial encounters (Cumberbatch et al. 1997; Banchereau & Steinman 1998; Flores-Romo 2001) is the appearance of activation, co-stimulatory or even maturation markers, we decided to assess two major representatives of such molecules: CD80 and CD83. The co-stimulatory molecule CD80 is normally absent in resting epidermal LC (Figure 5e), but cutaneous DV infection apparently alters this pattern inducing its expression (Figure 5f). CD83 is a maturation marker for human DC and, therefore, is not expressed in normal unperturbed conditions, nor in peripheral immature DC such as epidermal LC (Figure 5a). Interestingly, CD83 expression was clearly induced in DV-infected skin explants (Figure 5b–d, g, arrows in b). However, by performing labelling in sequential sections for DV, it appears that the induction of CD83 expression (Figure 5g) does not seem to match with the scarce positivity found for DV (Figure 5h, arrows). This would seem to indicate that, although the tissue microenvironment of the virally infected skin does induce this maturation molecule, its expression might not be induced in the currently infected cells. Alternatively, the possibility exists that the very few epidermal DCs which become infected might somehow be precluded to reach maturation and do not express CD83.
Figure 5.
Infection with new dengue virus 2 (DV2) induces the appearance of (activation) CD80 and (maturation) CD83 molecules in epidermis. Conventional skin sections of DV2-infected explants (b–d, f–h) revealed the emergence of CD83+-mature dendritic cell (DC) (blue labelling in b-d, g and arrows in b) and of CD80+-activated DC (blue labelling in f) confined to the epidermis, but without apparent correspondence to the scant and weak DV positivity found (faint blue labelling in h, arrows) in skin serial sections of DV-inoculated explants. (c) represents a magnification of b, while d is a magnification of another skin explant. No CD83+-mature DCs were found in the dermis of DV2-infected explants (b, c, d, g). Absence of mature CD83+ DC and of CD80 is also observed in control-cultured non-infected explants (a and e, respectively). Pictures were taken at 5 days after culture initiation and are representatives of five skin samples from different individuals. Dotted line (b) indicates the dermo-epidermal junction, and brownish colour is due to melanin+ cells in these skin types.
In situ apoptosis upon local DV infection of skin explants
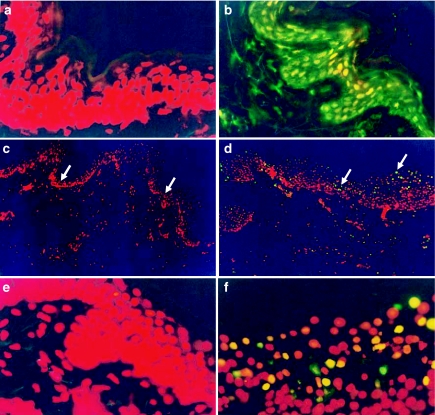
Because many viruses induce apoptosis upon infection, and this has been shown for flavivirus, including DV in human necropsies, we evaluated whether this type of cell-death process would occur in situ upon local DV2 inoculation into the skin. As shown in Figure 6d, f, apoptosis was clearly induced and evident in DV2-infected skin explants by comparison with control non-infected skin cultures (Figures 6c, e). TUNEL (green fluorescence)-positive cells were abundant mostly in the outer part of epidermis of DV-infected explants (Figure 6d arrows). Although we have not yet assessed which specific cells are driven into apoptosis, due to both the abundance and the histological location of TUNEL positivity, it is highly likely that these cells are mostly keratinocytes.
Figure 6.
Cutaneous dengue virus 2 (DV2) infection induces apoptosis (TUNEL positivity) mainly in the epidermis. The TUNEL technique was used to assess apoptotic cell death following DV2 infection. (a) shows TUNEL-negative control-skin explants (no Dnase), whereas (b) illustrates TUNEL-positive control sample treated with Dnase, indicated by the green fluorescence. (c) shows TUNEL results in skin from control-cultured non-infected explants, whereas (d) shows skin from DV2-infected explants. (e) represents a magnification of (c), while (f) is a magnification of (d). Arrows in (d) indicate the outer part of epidermis where TUNEL positivity is more apparent in DV-infected skin, whereas arrows in (c) illustrate the lack of apoptosis in the corresponding areas of control skin. Results are representative of three different experiments in samples from three different subjects.
Discussion
Although excellent research about DV has been stimulated in recent years by the resurgence of dengue worldwide, this has been mostly dedicated to the molecular and the epidemiological aspects of this arboviral infection (Leitmeyer et al. 1999; Valdes et al. 2000; Kuhn et al. 2002; Modis et al. 2003; Welsh & Rothman 2003; Gomez-Dantes et al. 2004). By contrast, research to elucidate the basic underlying mechanisms leading to the pathology of human DV infection is rather limited. It is well known that DV enters via skin by mosquito biting while feeding; however, both the very early stages of DV infection and the initial local encounters between DV and elements of the immune system in situ remain largely unexplored. Because, by necessity, patients are examined when disease is almost over, when they are under recovery or when they have entered into DSS, perhaps, this has precluded the study of the earliest phases of DV infection and of the potential responses in situ.
We consider that studying the cutaneous DCs is important, because these are the most exposed cells of the (innate) immune system, which, by histological position and functions, are likely to interact first with the initial viral load while female mosquitoes feed. Because of these mosquitoes' feeding habits, the proboscis must traverse several times (‘probing’) the epidermis before reaching a blood vessel in the dermis. In the process, the salivary glands release the virus. Conceivably, viral particles are likely to interact first with cells of the various epidermal layers (Putnam & Scott 1995; Platt et al. 1997).
However, there are very few reports of cutaneous DCs and DV. One study in mice (Taweechaisupapong et al. 1996) describes increased LC numbers upon skin DV inoculation, but this would seem to differ from most reports about cutaneous stimuli, where murine LC density decreases (Banchereau & Steinman 1998; Flores-Romo 2001). There are studies with human DCs, but these were generated in vitro from monocyte precursors (Libraty et al. 2001) and showed that these DCs are at least 10 times more permissive for DV infection in culture than monocytes (Wu et al. 2000); these latter cells are considered so far the primary target cells for DV. Recently, DV infection (Wu et al. 2000) was described in human skin DC, but this study has four major differences with ours: firstly, they used cadaveric (not fresh) skin from 12 h after death; secondly, they infected the DC coming out from the cultures (not the DC infected in situ); thirdly, they assessed the infection by means of antibodies (and not by the intermediate viral RNA) and fourthly, they used an attenuated DV-tetravalent vaccine to inoculate the skin of one subject (we used a virulent DV2 clinical isolate for all the explants).
The five skin samples infected were from Mexico City residents. Because of the geographical location, especially an altitude of 2240 m above the sea level, dengue is uncommon in Mexico City and usually imported. Because we did not add anti-DV+ sera nor anti-DV antibodies to the skin explants, we would like to suggest that our model might be more appropriate to study the primary contacts with DV, where no facilitating antibodies are yet involved. Likewise, although the number of skin samples tested is rather small for wider conclusions, and because none of the subjects were relatives, but all samples were nonetheless infected, this would suggest that perhaps infection with this DV2 isolate is not associated to an MHC restriction. However, a strict haplotype characterization as well as larger number of samples will be required to better sustain this point.
Viruses are known for using a variety of sophisticated mechanisms to evade or divert immune responses away (Bachmann et al. 1998; Zinkernagel & Hengartner 2001). One strategy frequently used by viruses, including flaviviruses like dengue, is the induction of apoptotic cell death, which was clearly induced in situ in the epidermis of DV-infected skin explants. Although we did not address the issue of exactly which cells within the epidermis were driven into apoptosis, both the frequency and the histological location of TUNEL+ cells point most likely to keratinocytes as the apoptotic targets of early DV infection in situ. It should also be noted that the main bulk of apoptotic (green fluorescent) cells was located in the outer part of epidermis, a skin subregion where DV+ cells were usually not found, suggesting perhaps an indirect effect. In postmortem samples, DV replication has been reported in several tissues (Jessie et al. 2004). However, we believe this is the first report of in situ DV infection of non-haemopoietic cutaneous cells such as the keratinocyte, and also of apoptosis in these cells as a consequence of this viral infection in the skin. Therefore, in this model, DV seems an effective microbial threat to the skin-cell populations, manifested by its presence in keratinocytes and by the induction of apoptotic death, by triggering derangements of both the morphology and the well-ordered spatial distribution of LC in situ, as well as by decreasing the LC frequency.
Because some of the earliest manifestations seen after DCs have encountered potential antigenic threats (either from chemical, microbial or from other sources), for instance, the appearance of activation or co-stimulatory molecules such as members of the B7 family, we explored whether cutaneous DV inoculation would be associated to the expression of two prominent members of this group: CD80 and CD83. CD80 is one of the three major co-stimulatory molecules in APCs, that during an immune response interacts with CD28, one of its counterparts in activated T cells. CD80 is thus a major component of the current two-signal paradigm for T-cell activation. Very recently, however, there has been the proposition that, because CD80 is a dimer and binds to CTLA-4 (CD152), the outcome of this latter interplay might also be tolerance or suppression, not only the activation of T cells (Sansom et al. 2003). Interestingly, CD80+ cells appeared in close proximity to the basal layer of keratinocytes in epidermis, where LC would normally reside, while CD83+ cells seemed located mostly towards the external part of the epidermis, without apparent correspondence with the CD80+ cells. CD83 is perhaps the best-established maturation marker for human DC, which is normally absent in resting, peripheral DC such as epidermal LC. Although CD83 might be found in other cells, at least regarding the skin, there is no evidence that other cells than the DCs would express this molecule. Of note, CD83 expression was clearly induced in the epidermis upon DV infection. However, at least two points deserve a comment in this respect: (i) CD83+ cells were mostly observed towards the outer part of epidermis, a rather unusual and intriguing finding, because LC, when migrating (ensuing activation), do it in the opposite direction, towards the dermis and (ii) When performing sequential immunolabelling with CD83 and DV, we consistently found that although epidermal DV+ cells were rather scarce, there was no apparent coincidence with the more evident CD83+ cells, suggesting that DV-induced maturation of LC does not seem to occur in the infected cells. Although in different settings, our results concur in general with previous studies using monocyte-derived DC, cadaveric skin DC and an attenuated DV vaccine inoculation into skin (Wu et al. 2000; Libraty et al. 2001). In these reports, DV infection was also found restricted to immature DC.
We believe that another interesting observation in our study is the fact that the emerging CD83+ (maturing) DCs were never observed in the dermis (Figure 5), rather intriguingly, they seemed located mostly in the outer part of the epidermis. To us, this suggests that the maturation effect (CD83 induction) of cutaneous DV infection would seem uncoupled from another very crucial DC function, the mobilization into dermis. In fact, by electron microscopy, human DCs have been described forming well-ordered ‘DC cords’ as they migrate into the dermis (Cumberbatch & Kimber 1992; Cumberbatch et al. 1997; Romani et al. 2001). This opens the possibility that these findings might represent new viral subversion strategies, yet undescribed for DV, especially in relation to human cutaneous DC. However, findings such as these will be rather difficult to observe, unless the experiments are performed in situ using whole skin.
We believe these are obviously intriguing phenomena, worthy of further research especially regarding the subsequent T-cell stimulation by these activated/mature DCs, which would seem not to bear the virus themselves.
In summary, we hope that this model provides an experimental tool to incite researchers to further explore the early, basic mechanisms of DV–host immune system interactions in situ. This approach is quick (48 h), rather inexpensive (microscope, PCR and histology facilities) and thus feasible to be performed in most laboratories, especially in the settings of developing countries where dengue infection has increased in the last decade (WHO 1997). Additional advantages include the fact that, because the skin is derived from healthy women undergoing plastic surgery, it is a de facto biological waste from non-infectious origin thus not posing a biological hazard for manipulations in the laboratory, and also requiring less-strict ethical compliances. Another important advantage is that we have used freshly obtained non-cadaveric skin and have assessed the DV replication by means of both the intermediate viral RNA and NS1, unlike one previous report where cadaveric skin samples from 12 to 24 h were employed (Wu et al. 2000). Because the assay is relatively quick, inexpensive and not too complex, it is also envisaged as a tool to assess compounds with potential effects upon virus binding, uptake or replication, as well as substances with potential antiviral effects.
Acknowledgments
Authors thank the laboratory of virology, especially Fernando Medina-Ramirez for helping with insect cell cultures, also the laboratories from INDRE, ENCB, Cellular Immunology and Molecular Biomedicine and Dr C. Cruz-Ceron for all help provided. A Y Limon-Flores, M Perez-Tapia, A Brizuela-Garcia, S E Herrera-Rodriguez, M Heras-Chavarria and A Flores-Langarica are fellow holders from the National Council for Science and Technology CONACYT. I Estrada-Garcia, A Escobar-Gutierrez, L Cedillo-Barron and L Flores-Romo are SNI members.
References
- Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161:6338–6346. [PubMed] [Google Scholar]
- Bachmann MF, Zinkernagel RM, Oxenius A. Immune responses in the absence of costimulation: viruses know the trick. J Immunol. 1998;161:5791–5794. [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Cumberbatch M, Kimber I. Dermal tumour necrosis factor-alpha induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans' cell migration. Immunology. 1992;75:257–263. [PMC free article] [PubMed] [Google Scholar]
- Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology. 1997;92:388–395. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, Harris E. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol. 2000;74:4957–4966. doi: 10.1128/jvi.74.11.4957-4966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Romo L. In vivo maturation and migration of dendritic cells. Immunology. 2001;102:255–262. doi: 10.1046/j.1365-2567.2001.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Dantes H, Ibañes-Bernal S, Valedspino-Gomez J, Velasco-Castrejon O, Escobar-Gutierrez A, Magos-Lopez C. Dengue in Enfermedades Tropicales en Mexico. México D.F.: INDRE, SSA.; 2004. pp. 85–96. [Google Scholar]
- Gould EA, Clegg JCS. Growth, titration and purification of alphaviruses and flaviviruses. In: Mahy BWJ, editor. Virology: a practical approach. Oxford: IRL Press; 1991. p. 437. [Google Scholar]
- Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB, O'Rourke EJ, Allison AC. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977;146:218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- Haywood AM. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- Johnston LJ, Halliday GM, King NJ. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Invest Dermatol. 2000;114:560–568. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi I, Sasaki A, Boots M. Why are dengue virus serotypes so distantly related? Enhancement and limiting serotype similarity between dengue virus strains. Proc Biol Sci. 2003;270:2241–2247. doi: 10.1098/rspb.2003.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P, Zinkernagel RM. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394:482–485. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- Kuhn RJ, Zhang W, Rossmann MG, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitmeyer KC, Vaughn DW, Watts DM, et al. Dengue virus structural differences that correlate with pathogenesis. J Virol. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libraty DH, Pichyangkul S, Ajariyakhajorn C, Endy TP, Ennis FA. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J Virol. 2001;75:3501–3508. doi: 10.1128/JVI.75.8.3501-3508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci USA. 2003;100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolsapaya J, Dejnirattisai W, Xu XN, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- Morens DM. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin Infect Dis. 1994;19:500–512. doi: 10.1093/clinids/19.3.500. [DOI] [PubMed] [Google Scholar]
- Platt KB, Linthicum KJ, Myint KS, Innis BL, Lerdthusnee K, Vaughn DW. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg. 1997;57:119–125. doi: 10.4269/ajtmh.1997.57.119. [DOI] [PubMed] [Google Scholar]
- Putnam JL, Scott TW. The effect of multiple host contacts on the infectivity of dengue-2 virus-infected Aedes aegypti. J Parasitol. 1995;81:170–174. [PubMed] [Google Scholar]
- Raengsakulrach B, Nisalak A, Maneekarn N, et al. Comparison of four reverse transcription-polymerase chain reaction procedures for the detection of dengue virus in clinical specimens. J Virol Methods. 2002;105:219–232. doi: 10.1016/s0166-0934(02)00104-0. [DOI] [PubMed] [Google Scholar]
- Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- Romani N, Ratzinger G, Pfaller K, et al. Migration of dendritic cells into lymphatics-the Langerhans cell example: routes, regulation, and relevance. Int Rev Cytol. 2001;207:237–270. doi: 10.1016/s0074-7696(01)07007-3. [DOI] [PubMed] [Google Scholar]
- Sansom DM, Manzotti CN, Zheng Y. What's the difference between CD80 and CD86? Trends Immunol. 2003;24:314–319. doi: 10.1016/s1471-4906(03)00111-x. [DOI] [PubMed] [Google Scholar]
- Talavera D, Castillo AM, Dominguez MC, Gutierrez AE, Meza I. IL8 release, tight junction and cytoskeleton dynamic reorganization conducive to permeability increase are induced by dengue virus infection of microvascular endothelial monolayers. J Gen Virol. 2004;85:1801–1813. doi: 10.1099/vir.0.19652-0. [DOI] [PubMed] [Google Scholar]
- Taweechaisupapong S, Sriurairatana S, Angsubhakorn S, et al. Langerhans cell density and serological changes following intradermal immunisation of mice with dengue 2 virus. J Med Microbiol. 1996;45:138–145. doi: 10.1099/00222615-45-2-138. [DOI] [PubMed] [Google Scholar]
- Valdes K, Alvarez M, Pupo M, Vazquez S, Rodriguez R, Guzman MG. Human Dengue antibodies against structural and nonstructural proteins. Clin Diagn Lab Immunol. 2000;7:856–857. doi: 10.1128/cdli.7.5.856-857.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Rothman AL. Dengue immune response: low affinity, high febrility. Nat Med. 2003;9:820–822. doi: 10.1038/nm0703-820. [DOI] [PubMed] [Google Scholar]
- WHO. 1997. Dengue and Dengue Haemorrhagic Fever: diagnosis, treatment and control. http://www.who.int/csr/resources/publications/dengue/Denguepublication/en/
- Winkler G, Maxwell SE, Ruemmler C, Stollar V. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology. 1989;171:302–305. doi: 10.1016/0042-6822(89)90544-8. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Grouard-Vogel G, Sun W, et al. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Hengartner H. Regulation of the immune response by antigen. Science. 2001;293:251–253. doi: 10.1126/science.1063005. [DOI] [PubMed] [Google Scholar]