Abstract
Vascular endothelial growth factor (VEGF) plays a central role in tumour angiogenesis. In a mouse intramuscular tumour model using VEGF-transfected HT1080 human fibrosarcoma, we investigated the morphological features and patterns of remodelling in size-matched tumours. Compared with the control tumours (C group), the VEGF-transfected tumours (V group) showed vigorous neovascularization with larger vessels. Fenestrations and disruptions of endothelia were specific to the V group. Three types of vascular remodelling, i.e. sprouting, luminal division and intussusceptive microvascular growth, were present in both groups. Morphometric analyses revealed that mural cell coverage of the endothelial cells was significantly smaller in the V group compared with that in the C group (V group, 28.2 ± 18.6%; C group, 41.6 ± 21.1%; P < 0.0001). To determine the prevalence of remodelling patterns, the occurrences of abluminal and luminal processes on endothelial cell surfaces were quantified. Abluminal processes are defined as cytoplasmic protrusions of the abluminal membrane of endothelial cells, which can vary from tiny spurs to solid sprouts of the cell. On the other hand, luminal processes are defined as intraluminal protrusions of the endothelial cell membrane, including various membranous changes from filiform processes to rather thick cytoplasmic bulges. An abluminal process is thought to represent an initial morphological change in sprouting type angiogenesis, and a luminal process to be a sign of implementation of luminal division. The frequency of abluminal processes was significantly higher in the V group than in the C group (V group, 0.243 ± 0.138/µm; C group, 0.114 ± 0.101/µm; P < 0.0001). In contrast, the number of luminal processes on the endothelial cells per micrometre was statistically comparable between the groups (V group, 0.285 ± 0.252/µm; C group, 0.309 ± 0.236/µm, P = 0.381). These results indicate that sprouting is the main mode of VEGF-induced tumour angiogenesis.
Keywords: tumour angiogenesis, VEGF, remodelling, pericyte coverage, electron microscopy
As angiogenesis plays a critical role in tumour growth, a precise understanding of the morphological features as well as the molecular mechanisms involved in tumour angiogenesis is necessary to develop better therapeutic strategies.
Morphologically, two aspects are of great importance in angiogenesis: (1) the contact between pericytes and endothelial cells and (2) the types of vascular remodelling, i.e. sprouting, intussusception and luminal division.
There have been many reports suggesting that pericytes play an important role in angiogenesis and vascular maturity. Withdrawal of pericytes occurs prior to endothelial cell proliferation in an adult rat cardiac and skeletal muscle angiogenesis model, suggesting that pericytes exert an inhibitory effect on endothelial cell proliferation (Egginton et al. 1996). Similarly, pericyte coverage of the vascular endothelium brings vessel maturation and marks the end of the plastic state of immature vessels (Benjamin et al. 1998).
Currently, three distinct models of vascular morphogenesis and remodelling have been recognized in various settings of angiogenesis. Sprouting is the most conventional type of angiogenesis and most frequently used to evaluate angiogenesis both in vitro and in vivo. Capillary sprouts originate as small endothelial spurs, which eventually grow to form anastomoses with other sprouts and create functional capillary loops (Rhodin & Fujita 1989). The other two modes of angiogenesis basically involve intraluminal splitting of the blood vessels that expand the blood flow by increasing the number of blood vessels in situ. Intussusceptive microvascular growth (IMG) refers to vascular remodelling by insertion of tissue pillars or interstitial tissue structures (ITSs) into the vascular lumen, resulting in partitioning of the vessel lumen (Caduff et al. 1986; Patan et al. 1996a). Despite having a similar remodelling pattern to IMG, luminal division is another distinct type of intraluminal remodelling in that this does not require interstitial tissue intrusion from outside the vessel wall at the early phase of implementation. Luminal division begins with small intraluminal processes or vacuolization of endothelial cells without any morphological change of the abluminal surface that grows to divide the vessel lumen into segments (Zhou et al. 1998).
There are several factors that affect the pattern of angiogenesis. For example, the polarity of mechanical stimuli to the endothelial cells, i.e. intraluminal or abluminal stimulation, determines which mode of angiogenesis occurs (Egginton et al. 2001). In relation to growth factors, little is known as to which type of angiogenesis is preferentially induced under a certain kind of growth factor regime.
Vascular endothelial growth factor (VEGF) is a potent modulator of vascular endothelial cells. There are a vast amount of data reported to date, showing that VEGF acts as a vascular permeability factor as well as a mitogen and a survival factor of endothelial cells. Although it has been taken for granted that capillary sprouting is the prime mode of VEGF-induced angiogenesis, no such quantitative data have been published so far that compared the frequency of sprouting and other types of angiogenesis on the basis of the intensity of VEGF expression in the same tumour model.
Previously, we reported that VEGF promoted tumourigenicity and metastasis by inducing tumour angiogenesis in hepatocellular carcinoma and colorectal tumours (Mise et al. 1996; Ishigami et al. 2000; Kondo et al. 2000a) as well as in VEGF-overexpressing tumour cell models in mice (Mori et al. 1999; Kondo et al. 2000b). In the present study, focusing on the role of VEGF in the tumour angiogenesis, we evaluated the morphological interaction between endothelial cells and mural cells, and quantified the frequency of abluminal and luminal processes on the plasmalemmal membrane of endothelial cells that potentially lead to sprouting or luminal division in a VEGF-induced tumour angiogenesis model.
Methods
Cell culture and preparation
The human fibrosarcoma cell line HT1080 was transfected with pCAG-BSD-hVEGF121 or pCAG-BSD to generate stable transfectants and was cultured as described previously (Mori et al. 1999). Briefly, the full-length hVEGF121 cDNA (505 bp) was isolated from a human colon cancer specimen by reverse-transcriptase polymerase chain reaction. An eukaryotic expression vector pCAG-BSD was constructed from two parts of pCAGGS (Niwa et al. 1991) and pMAM2-BSD (Kaken, Tokyo, Japan). The hVEGF121 cDNA was cloned into the XhoI restriction site of pCAG-BSD to create the plasmid pCAG-BSD-VEGF, in which the transcription of VEGF was constitutively driven by the CAG-enhancer promoter, and the drug-resistant selection gene BSD (Blasticidin S deaminase) was present (Kimura et al. 1994). HT1080 cells were transfected with the pCAG-BSD-hVEGF121 or pCAG-BSD by Lipofectamine (Gibco-BRL, Gaithersburg, MD, USA) and the stable transfectants, HT1080-VEGF and HT1080-mock, were established. The expression levels of VEGF mRNA and the secreted VEGF protein were confirmed by Northern blot analysis and Western blot analysis, respectively. Of the nine clones of the HT1080-VEGF transfectant, the clone that showed the strongest expression of VEGF was labelled ‘V cell’, and was used as the VEGF transfectant in this study. Although both Northern blot and Western blot analysis detected a certain amount of expression of VEGF in the HT1080-mock transfectant (labelled ‘C cell’), the V cells produced additional amount of human VEGF both in vitro and in vivo. In this study, the exact values of the secreted VEGF by the tumour cells were not measured. The transfectants were cultured in minimum essential medium (MEM; Nissui, Tokyo, Japan) containing 10% heat-inactivated fetal bovine serum (BioWhittaker, Walkersville, MD, USA), 100 units/ml penicillin, 100 µg/ml streptomycin and 10 µg/ml Blasticidin S (Kaken) in a humidified atmosphere of 5% CO2. A subconfluent monolayer of each cell line was harvested with trypsin and washed twice in phosphate-buffered saline (PBS), and 1 × 107 cells were suspended in 0.2 ml of PBS for tumour injection.
Intramuscular tumour inoculation model
Five-week-old male BALB/cAnCrj-nu/nu mice were purchased from Charles River Japan (Yokohama, Japan). After 1 week of acclimatization, mice were given an intramuscular injection of the tumour cell suspension into the left hindlimb (six mice for the V group and five for the C group). The mice were sacrificed and the tumours were excised and prepared for further examination when they reached approximately 10 mm in diameter, which was 5–10 days after inoculation depending on the cell type. All in vivo experiments were performed in accordance with the Guidelines for Animal Experiments of Kyoto University.
Immunohistochemistry of tumour vessels
The peripheral regions of the tumours were cut into blocks and fixed in PBS containing 4% paraformaldehyde and processed for paraffin embedding. Sections of 5 µm thickness were cut and subjected to haematoxylin and eosin staining and immunohistochemistry. To visualize the tumour vessels, the sections were immunostained with antimouse CD34 monoclonal antibody (RAM34; PharMingen International, San Diego, CA, USA). In brief, the sections were deparaffinized and rehydrated in a descending series of ethanol. After antigen retrieval treatment by autoclaving in Target Retrieval solution (S1699, DAKO, Carpenteria, CA, USA) for 15 min and blocking of endogenous peroxidase with 3% hydrogen peroxide, the sections were incubated with rat antimouse CD34 monoclonal antibody (dilution;1:50) for 1 h at room temperature. Following the incubation with the second antibody (E0468; biotinylated rabbit polyclonal antirat antibody; DAKO; dilution 1:250) for 20 min, the sections were reacted with streptavidin peroxidase (DAKO CSA System) for 20 min. Diaminobenzidine (DAB) was used as chromogen and the sections were counterstained with haematoxylin.
Transmission electron microscopy
The excised tumour was superfused with 2.5% glutaraldehyde in 0.1 m PBS. The peripheral regions of the tumour were sliced into blocks with faces of 1–2 mm2 and fixed by immersion in the same fixative for 4 h at 4 °C. The blocks were then rinsed twice in PBS containing 0.2 m sucrose and postfixed in 1% osmium tetroxide, dehydrated in an ascending series of ethanol, cleared in propylene oxide and embedded in resin (Spurr Resin Embedding Kit, Taab, Berkshire, UK). At least 10 blocks per tumour were chosen at random, and semithin sections of 1 µm thickness were cut and stained with toluidine blue to lightmicroscopically orientate the tumour vessels in the block. The vascular-rich area of each block was further trimmed to ultrathin sections of 80-nm thickness and stained with uranyl acetate and lead citrate. The sections were observed with a transmission electron microscope (Hitachi, Ibaraki, Japan) at an accelerating voltage of 75 kV.
Morphometric quantitative analysis
Tumour microvessels with complete circumferences in cross-section that are smaller than 40 µm were photographed randomly by electron microscopy at a magnification of × 4000–12,000. On these photographs, three morphological aspects were quantified using a wire-measuring method. A wire was placed along the line to be measured, and the actual length was calculated in terms of the magnification. As it is not easy to identify cells in close apposition to a capillary as pericytes, those cells in close contact with endothelial cells are termed mural cells in this paper. Mural cell coverage was defined as the percentage of the abluminal circumference of the vessel covered by mural cells and calculated as (the length of the abluminal surface of the vessel directly covered by mural cells)/(the length of the abluminal circumference of the vessel) × 100. For the purpose of clarifying the predominant type of angiogenesis occurring in the sample, abluminal processes and luminal processes on the endothelial cell plasma membrane were counted and the frequency of occurrence on the circumference were calculated. Abluminal processes were defined as cytoplasmic protrusions of abluminal membrane of the endothelial cell, which varied from tiny spurs to solid sprouts of the cell. On the other hand, luminal processes were defined as intraluminal protrusions of the endothelial cell membrane including various membranous changes from filiform processes to rather thick cytoplasmic bulges. Abluminal processes are considered to be an initial morphological change in sprouting type angiogenesis (Rhodin & Fujita 1989), while luminal processes are thought to be a sign of implementation of luminal division (Zhou et al. 1998).
Statistical analysis
All results are expressed as mean ± standard deviation. Differences between groups were analysed by the unpaired Student's t-test or the χ2 test. P-values <0.05 were considered significant.
Results
Light microscopic observation of tumour vessels
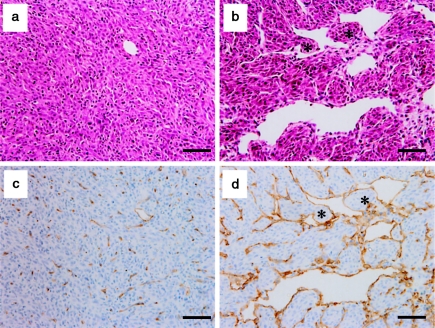
Haematoxylin and eosin staining and immunohistochemistry for CD34 revealed more vigorous and larger tumour vessels in the V group than in the C group. From a morphological point of view, it was noteworthy that 54% of the large tumour vessels in the V group (107/198 vessels of six tumours) featured tissue pillars or ITSs, which are landmarks of IMG, whereas in the C group, such structures were rarely seen, probably because of the lack of large vessels (Figure 1).
Figure 1.
Tumour vessels in HT1080 intramuscular tumours from the vascular endothelial growth factor-transfected (V) group and the mock-transfected (C) group. The peripheral regions of the tumours were observed by haematoxylin and eosin staining (a, b) and immunohistochemistry with anti-CD34 monoclonal antibody(c, d). The tumours from the V group showed a more vigorous and intense vascularization than the control tumours. In addition to the appearance of large sinusoidal vessels in the V group, immunostaining for CD34 revealed fine networks of tumour vessels, reflecting perpetuating tumour angiogenesis (d). Notably, interstitial tissue structures suggestive of intussusceptive microvascular growth were frequently present in large sinusoidal vessels in the V group (asterisks in b and d). a, c: tumours from C group; b, d: tumours from V group. Scale bars: 50 µm.
Transmission electron microscopic observation of tumour vessels
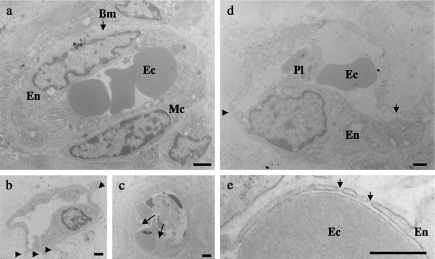
To further analyse the effects of VEGF on the morphological aspects of tumour angiogenesis, tumour vessels of each group were observed by transmission electron microscopy (TEM). The tumour vessels of the peripheral region, especially in the VEGF-transfected tumours, varied in size and shape; however, the basic components of the microvessel, i.e. vascular endothelial cells, mural cells and basement membrane remained in place in both groups. A close observation of endothelial cells revealed plasmalemmal processes on the abluminal and luminal surfaces. Particularly in the V group, endothelial fenestrations were frequently observed. Most of the vessels with fenestrations had elongated and sometimes disrupted endothelia. Another specific feature of tumour vessels in the V group was the appearance of enlarged nuclei indicative of activated endothelial cells (Figure 2).
Figure 2.
Transmission electron microphotographs of representative tumour microvessels. (a) Microvasculature from the control tumour showing intact vascular elements, i.e. endothelial cells, mural cells and basement membrane. (b) Abluminal processes emerging from a vascular endothelial growth factor (VEGF)-transfected tumour vessel (arrowheads). (c) Luminal processes originating from the inside wall of endothelial cells of a VEGF-transfected tumour vessel (arrows). (d) A characteristic microvessel observed in the VEGF-transfected tumour. Vascular endothelium was elongated with a plump nucleus, suggesting endothelial cell activation. Endothelial fenestrations (arrow) and a disruption (arrowhead) were specific to the VEGF-transfected tumours. (e) Higher magnification of the endothelial fenestrations with diaphragms (arrows). Bm, basement membrane; Ec, erythrocyte; En, endothelial cell; Mc, mural cell; Pl, platelet. Scale bars: 1 µm (a–d); 0.5 µm (e).
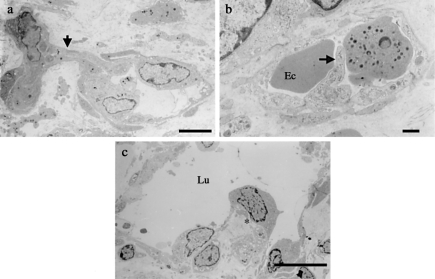
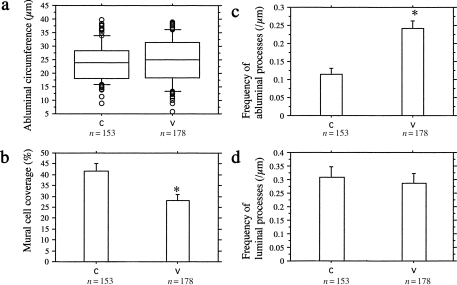
As for the types of angiogenesis, i.e. sprouting, luminal division and IMG, all three modes were observed in the tumours from both groups (Figure 3). As described in the light microscopic observation, the fact that IMG occurred only in large vessels hampered the comparison of the frequency of IMG between the two groups due to the scarcity of large tumour vessels in the C group. The length of abluminal circumference of the vessels analysed in this study was comparable between the groups (V group, 24.9 ± 8.0 µm; C group, 24.0 ± 6.9 µm, P = 0.27). In these small tumour vessels, endothelial fenestrations were present in 22.5% (40/178) of the vessels in the V group and 1.3% (2/153) in the C group (P < 0.0001). Mural cell coverage of the endothelial cells was significantly smaller in the VEGF-transfected tumours compared to that in the control tumours (V group, 28.2 ± 18.6%; C group, 41.6 ± 21.1%; P < 0.0001). The number of abluminal processes on the endothelial cell surface per micrometre was significantly higher in the VEGF-transfected tumours than in the control tumours (V group, 0.243 ± 0.138/µm; C group; 0.114 ± 0.101/µm; P < 0.0001). In contrast, the number of luminal processes on the luminal surface of endothelial cells per µm was comparable between the groups (V group, 0.285 ± 0.252/µm; C group, 0.309 ± 0.236/µm; P = 0.381) (Figure 4).
Figure 3.
Three types of tumour angiogenesis observed in the vascular endothelial growth factor (VEGF)-transfected tumours. (a) Sprouting angiogenesis showing a sprout (arrow) with a slit-like lumen originating from two endothelial cells. (b) Luminal division of a tumour vessel with a luminal process (arrow) separating the vessel lumen. (c) Intussusceptive microvascular growth (IMG) was observed in large sinusoidal vessels in the VEGF-transfected tumours. The asterisk indicates interstitial tissue structures that are hallmarks of IMG. IMG was rarely seen in the control tumours probably because of the lack of large vessels. Ec, erythrocyte; Lu, lumen. Scale bars: 5 µm (a); 1 µm (b); 10 µm (c).
Figure 4.
Quantitative analyses on the morphological changes in the tumour microvessels. (a) The length of abluminal circumference of the vessels analysed in this study was smaller than 40 µm, and there was no significant difference between the groups. (b) The percentage of mural cell coverage of endothelial cells was significantly lower in the vascular endothelial growth factor (VEGF)-transfected tumours than the control tumours. (c) The frequency of abluminal processes on endothelial cells per µm was significantly higher in the VEGF-transfected tumours than the control tumours. (d) The difference in the frequency of luminal processes was not significant. *P < 0.0001.
Discussion
Tumour vessels are highly heterogenous, both morphologically and biologically within individual tumours as well as among different tumours (Paku & Lapis 1993; Eberhard et al. 2000). Our knowledge about the initiation of tumour neovascularization has become complex as many concepts, such as vasculogenic mimicry, vessel co-option and involvement of endothelial progenitor cells in solid tumour angiogenesis, have emerged over the last decade (Asahara et al. 1999; Holash et al. 1999; Maniotis et al. 1999). In addition, the coexistence of various stages of angiogenesis and different modes of remodelling in a single tumour makes analysis of tumour vasculature quite difficult, therefore only a few studies have so far presented definite quantitative data on the tumour vessel morphology. In the present study, to mitigate the complexity of tumour vasculature, we only investigated the peripheral tumour vasculature, which is less affected by factors such as hypoxia, interstitial pressure and metabolites that are often enhanced in the tumour centre (Wartenberg et al. 2001). In addition, we sampled size-matched tumours around 10 mm in diameter where all kinds of remodelling are considered to be present and observed only small vessels whose perimeter in cross section was up to 40 µm. These observation limitations validated the morphometric and quantitative analyses on morphological changes and remodelling patterns in a VEGF-enhanced tumour angiogenesis model.
Basically, three essential components of the vascular walls, including endothelial cells, mural cells and the basement membrane, were found intact for the most part in both the VEGF-transfected and control tumours. Nevertheless, minor morphological differences existed between the groups. Plump nuclei of endothelial cells and enlarged vessel profile, for example, were frequently observed in the VEGF-transfected tumours. Among several morphological features induced by VEGF stimulation, induction of endothelial fenestration was very specific to the VEGF-transfected tumours, which is consistent with other reports (Roberts & Palade 1995, 1997; Esser et al. 1998; Feng et al. 1999a; Funyu et al. 2001; Chen et al. 2002). In this study, we observed that the fenestrae were always located in extremely thin endothelia, and that occasionally the vessel walls were found to be disrupted. Attenuated endothelial walls induced by VEGF may facilitate the generation and maintenance of fenestration. Recently, it has been shown that VEGF-induced vascular permeability increase is attributed to the coalescence and reorganization of caveolae resulting in vesiculovacuolar organelles (VVOs), trans-endothelial cell pores and fenestrae that provide a route for macromolecular substances to translocate through the endothelial wall bidirectionally (1999a Feng et al.1999a,b; Chen et al. 2002). Together with our results, vascular permeability increase in VEGF-expressing tumours seems to be caused by vesicle-related structures such as fenestration and/or endothelial disruptions.
In the present study, we demonstrated the three distinct types of tumour angiogenesis, i.e. sprouting, luminal division and IMG, in an intramuscular tumour model of HT1080 in nude mice. Furthermore, we investigated the ultrastructural changes in relation to tumour angiogenesis and clarified by comparing VEGF transfectants with control tumours that VEGF induces capillary sprouting rather than luminal division in small vessels. In addition, we found that the mural cell coverage in the VEGF-transfected tumours was lower than that in the control tumours, which also supports the hypothesis that VEGF facilitates sprouting.
Alternative splicing of human VEGF mRNA gives rise to at least six isoforms consisting of 121, 145, 165, 183, 189 and 206 amino acid residues (Zhang et al. 2000). With exception of VEGF121, these isoforms bind to heparin or heparan sulphate proteoglycan, which confers onto VEGF121 its most diffusable characteristic (Küsters et al. 2003). Many clinical studies have demonstrated that in various cancers, VEGF121, VEGF165, and VEGF189 are the main isoforms and that the expression and function of these isoforms vary substantially. High expression levels of VEGF121 mRNA reportedly are associated with some clinical features such as short survival, early relapse and high intratumoural microvessel density in renal cell carcinoma, nonsmall-cell lung carcinoma and breast carcinoma (Yuan et al. 2001; Kim et al. 2002; Ljungberg et al. 2003). In osteosarcoma, on the other hand, VEGF165 and VEGF189, but not VEGF121, were found to be essential for neovascularization (Lee et al. 1999). Likewise, some contradictory findings have been made by animal experiments regarding the functional difference of the isoforms. VEGF121-transfected MCF-7 breast carcinoma cells were more angiogenic and tumourigenic than were VEGF165 and VEGF189 transfectants (Zhang et al. 2000), while a melanoma-transfectant model VEGF165 or VEGF189 expression induced intratumoural neovascularization, but VEGF121 did not (Küsters et al. 2003). It was found that in a VEGF-deficient oncogenically transformed fibroblast cell line, only VEGF164 transfection (the murine version of VEGF165) could fully rescue tumour growth and neovascularization. In this model, VEGF120 (the murine version of VEGF121) induced peripheral neovascularization due to the diffuse signal perfusion (Grunstein et al. 2000). In our study, we used a VEGF121-transfected tumour model. The wild-type and mock-transfected HT1080 cells express endogenous VEGF121 and VEGF165 (Mori et al. 1999). Thus, the significance of this experimental model is to specify the tumour angiogenesis when VEGF121 is highly enhanced and co-ordinates with VEGF165. On the basis of these reported findings, we speculate that the biological function of VEGF in vivo is the result of co-ordination of various VEGF isoforms, leading us to believe that this model represents one aspect of actual tumour angiogenesis.
Although a number of in vitro studies have clearly demonstrated that VEGF induces angiogenic sprouting (Helmlinger et al. 2000; Korff et al. 2001), there has been only a few reports so far that has evaluated sprouting with respect to VEGF expression in tumours. Likewise, only a few studies have addressed the relationship between growth factors and patterns of vascular remodelling, e.g. VEGF and IMG (Wilting et al. 1996), Angiopoietin-1/TIE2 and IMG (Patan 1998). On the other hand, as for physical stimulation to the vessel endothelia, luminal division is induced by shear stress, whereas capillary sprouting is caused by abluminal stress in a physiological angiogenesis model in rats (Egginton et al. 2001).
In the present study, we provided evidence that VEGF generated by tumour cells induces microvascular sprouting rather than luminal division in vivo. In addition, although the frequency of IMG could not be compared between the groups due to the scarcity of large vessels in the control tumours, quite a few ITSs were observed in large vessels in the VEGF transfectants, suggesting that VEGF plays an important role in IMG as well as in sprouting. IMG, indeed, is a type of angiogenic remodelling that eventually separates a vessel lumen, thus resembling luminal division. However, quintessentially, the cells most involved in IMG especially in the early phase of remodelling are mesenchymal cells that lie abluminal to vessels (Burri & Tarek 1990; Patan et al. 1996a). Therefore, luminal division and IMG are quite different in the process, and IMG could be characterized, in a sense, as an abluminal type of angiogenesis that has an inward directionality. As observed in the present study, sprouting and IMG could be the main processes of tumour angiogenesis. In fact, the coexistence of IMG and sprouting has been reported in an LS174T human colon adenocarcinoma model (Patan et al. 1996b, 2001a). In the article, the authors observed that vascular growth occurred by both IMG and sprouting at the leading edge of the tumour. On the other hand, luminal division in tumour angiogenesis has been documented in a couple of papers in which luminal division is termed as transluminal endothelial bridging or vessel segmentation (Nagy et al. 1995; Patan et al. 2001a). As tumour-derived VEGF does not induce luminal division, it may be induced by other growth factors or shear stress generated by increased blood flow in tumours.
The interaction between vascular endothelial cells and mural cells has been a matter of interest for several decades (Shepro & Morel 1993). In physiological models of adult rat cardiac and skeletal muscle angiogenesis, the pericyte coverage area of vascular endothelia was reduced by physical angiogenic stimuli (Egginton et al. 1996). On the other hand, the degree of pericyte recruitment in a variety of human tumours appears to vary substantially (Eberhard et al. 2000). In the current study, the overexpression of VEGF caused a significant reduction of the mural cell coverage. There are two plausible explanations for this phenomenon. First, VEGF serves as a survival factor for endothelial cells (Reinmuth et al. 2001), therefore more endothelial cells could survive without pericyte coverage in the VEGF-transfected tumours, leading to a higher population of endothelial cells with a relatively smaller degree of pericyte coverage. Second, VEGF stimulated endothelial cells to upregulate Angiopoietin-2, resulting in the separation of pericytes from the vessel walls. However, in an in vitro experiment using cocultured spheroids of endothelial cells and smooth muscle cells, exogenous administration of VEGF did not affect the relative levels of Angiopoietin-1 and Angiopoietin-2 of the endothelial cells (Korff et al. 2001). Taken together, although the mechanism of VEGF-induced reduction of mural cell coverage still remains to be elucidated, it is most likely that abundant VEGF in concert with the reduction of pericyte coverage accelerated the proliferation of endothelial cells and contributed to the formation of capillary sprouting.
We reported previously that the VEGF-transfected tumours grew faster than the wild type in a subcutaneous tumour model (Mori et al. 1999). In that report, it was speculated that the growth advantage of the VEGF transfectants was attributed to the enhanced tumour vascularity as well as the suppression of apoptosis in the tumour cells. Also, in the current study, we observed the VEGF transfectants grew faster in the intramuscular tumour model, even though the number of inoculated cells and the injection site were different from those in the subcutaneous model. We speculate that the same mechanism is at work in this model.
The tumours analysed in this study were sampled on day 5 for the V group, and on day 10 for the C group to obtain samples from tumours of an appropriate size. It is therefore possible that the differences in the morphological features between the groups were the result of the difference in the times at which the tumours were sampled. However, additional studies comparing day 5 and day 10 tumours from both groups showed that the findings were very similar for the two time points (data not shown). Hence, we conclude that the data in our study were not affected by the time factor.
In conclusion, this study demonstrated the morphological characteristics of VEGF-induced tumour angiogenesis. Most importantly, we showed the three types of remodelling in a single tumour and presented clear evidence that VEGF promotes sprouting rather than luminal division and reduces the mural cell coverage. The vast majority of cancers express VEGF (Kerbel 2000). Moreover, we reported that the VEGF expression in tumours promotes tumour progression and metastasis by enhancing angiogenesis, leading to poor survival in many clinical settings as well as in animal experiments (Mise et al. 1996; Ishigami et al. 2000; Mori et al. 1999; Kondo et al. 2000a b). We therefore believe that the findings presented here provide a rationale for anti-angiogenic cancer therapies that inhibit tumour vessel sprouting by blocking the degradation of the extracellular matrix or the withdrawal of pericytes.
Acknowledgments
We thank Michiharu Kurino (Laboratory of Pathology, Kyoto University Hospital) for technical assistance with electron microscopy.
References
- Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- Burri PH, Tarek MR. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat. Rec. 1990;228:35–45. doi: 10.1002/ar.1092280107. [DOI] [PubMed] [Google Scholar]
- Caduff JH, Fischer LC, Burri PH. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat. Rec. 1986;216:154–164. doi: 10.1002/ar.1092160207. [DOI] [PubMed] [Google Scholar]
- Chen J, Braet F, Brodsky S, et al. VEGF-induced mobilization of caveolae and increase in permeability of endothelial cells. Am. J. Physiol. Cell. Physiol. 2002;282:C1053–C1063. doi: 10.1152/ajpcell.00292.2001. [DOI] [PubMed] [Google Scholar]
- Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–1393. [PubMed] [Google Scholar]
- Egginton S, Hudlicka O, Brown MD, Graciotti L, Granata AL. In vivo pericyte–endothelial cell interaction during angiogenesis in adult cardiac and skeletal muscle. Microvasc. Res. 1996;51:213–228. doi: 10.1006/mvre.1996.0022. 10.1006/mvre.1996.0022. [DOI] [PubMed] [Google Scholar]
- Egginton S, Zhou AL, Brown MD, Hudlicka O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc. Res. 2001;49:634–646. doi: 10.1016/s0008-6363(00)00282-0. 10.1016/S0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J. Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Nagy JA, Pyne K, Hammel I, Dvorak HF, Dvorak AM. Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation. 1999a;6:23–44. 10.1038/sj.mn.7300055. [PubMed] [Google Scholar]
- Feng Y, Venema VJ, Venema RC, Tsai N, Behzadian MA, Caldwell RB. VEGF-induced permeability increase is mediated by caveolae. Invest. Ophthalmol. Vis. Sci. 1999b;40:157–167. [PubMed] [Google Scholar]
- Funyu J, Mochida S, Inao M, Matsui A, Fujiwara K. VEGF can act as vascular permeability factor in the hepatic sinusoids through upregulation of porosity of endothelial cells. Biochem. Biophys. Res. Commun. 2001;280:481–485. doi: 10.1006/bbrc.2000.4148. 10.1006/bbrc.2000.4148. [DOI] [PubMed] [Google Scholar]
- Grunstein J, Masbad JJ, Hickey R, Giordano F, Johnson RS. Isoforms of vascular endothelial growth factor act in a coordinate fashion To recruit and expand tumor vasculature. Mol. Cell. Biol. 2000;20:7282–7291. doi: 10.1128/mcb.20.19.7282-7291.2000. 10.1128/MCB.20.19.7282-7291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmlinger G, Endo M, Ferrara N, Hlatky L, Jain RK. Formation of endothelial cell networks. Nature. 2000;405:139–141. doi: 10.1038/35012132. 10.1038/35012132. [DOI] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Ishigami SI, Arii S, Furutani M, et al. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br. J. Cancer. 2000;78:1379–1384. doi: 10.1038/bjc.1998.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- Kim SW, Park SS, Ahn SJ, et al. Identification of angiogenesis in primary breast carcinoma according to the image analysis. Breast Cancer Res. Treat. 2002;74:121–129. doi: 10.1023/a:1016150213253. 10.1023/A:1016150213253. [DOI] [PubMed] [Google Scholar]
- Kimura M, Takatsuki A, Yamaguchi I. Blasticidin S deaminase from Aspergillus terreus (BSD): a new drug resistance gene for transfection of mammalian cells. Biochim. Biophys. Acta. 1994;1219:653–659. doi: 10.1016/0167-4781(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Arii S, Furutani M, et al. Implication of vascular endothelial growth factor and p53 status for angiogenesis in noninvasive colorectal carcinoma. Cancer. 2000a;88:1820–1827. [PubMed] [Google Scholar]
- Kondo Y, Arii S, Mori A, Furutani M, Chiba T, Imamura M. Enhancement of angiogenesis, tumor growth, and metastasis by transfection of vascular endothelial growth factor into LoVo human colon cancer cell line. Clin. Cancer Res. 2000b;6:622–630. [PubMed] [Google Scholar]
- Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15:447–457. doi: 10.1096/fj.00-0139com. 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- Küsters B, de Waal RM, Wesseling P, et al. Differential effects of vascular endothelial growth factor A isoforms in a mouse brain metastasis model of human melanoma. Cancer Res. 2003;63:5408–5413. [PubMed] [Google Scholar]
- Lee YH, Tokunaga T, Oshika Y, et al. Cell-retained isoforms of vascular endothelial growth factor (VEGF) are correlated with poor prognosis in osteosarcoma. Eur. J. Cancer. 1999;35:1089–1093. doi: 10.1016/s0959-8049(99)00073-8. 10.1016/S0959-8049(99)00073-8. [DOI] [PubMed] [Google Scholar]
- Ljungberg B, Jacobsen J, Haggstrom-Rudolfssson S, Rasmuson T, Lindh G, Grankvist K. Tumour vascular endothelial growth factor (VEGF) mRNA in relation to serum VEGF protein levels and tumour progression in human renal cell carcinoma. Urol. Res. 2003;31:335–340. doi: 10.1007/s00240-003-0346-x. 10.1007/s00240-003-0346-x. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Folberg R, Hess A, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mise M, Arii S, Higashituji H. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology. 1996;23:455–464. doi: 10.1053/jhep.1996.v23.pm0008617424. [DOI] [PubMed] [Google Scholar]
- Mori A, Arii S, Furutani M, et al. Vascular endothelial growth factor-induced tumor angiogenesis and tumorigenicity in relation to metastasis in a HT1080 human fibrosarcoma cell model. Int. J. Cancer. 1999;80:738–743. doi: 10.1002/(sici)1097-0215(19990301)80:5<738::aid-ijc18>3.0.co;2-7. 10.1002/(SICI)1097-0215(19990301)80:5<738::AID-IJC18>3.3.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Masse EM, Herzberg KT, et al. Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res. 1995;55:360–368. [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. 10.1016/0378-1119(91)90434-D. [DOI] [PubMed] [Google Scholar]
- Paku S, Lapis K. Morphological aspects of angiogenesis in experimental liver metastases. Am. J. Pathol. 1993;143:926–936. [PMC free article] [PubMed] [Google Scholar]
- Patan S. TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc. Res. 1998;56:1–21. doi: 10.1006/mvre.1998.2081. 10.1006/mvre.1998.2081. [DOI] [PubMed] [Google Scholar]
- Patan S, Haenni B, Burri PH. Implementation of intussusceptive microvascular growth in the chicken chorioallantoic membrane (CAM): 1. pillar formation by folding of the capillary wall. Microvasc. Res. 1996a;51:80–98. doi: 10.1006/mvre.1996.0009. 10.1006/mvre.1996.0009. [DOI] [PubMed] [Google Scholar]
- Patan S, Munn LL, Jain RK. Intussusceptive microvascular growth in a human colon adenocarcinoma xenograft: a novel mechanism of tumor angiogenesis. MicrovascRes. 1996b;51:260–272. doi: 10.1006/mvre.1996.0025. 10.1006/mvre.1996.0025. [DOI] [PubMed] [Google Scholar]
- Patan S, Munn LL, Tanda S, Roberge S, Jain RK, Jones RC. Vascular morphogenesis and remodeling in a model of tissue repair: blood vessel formation and growth in the ovarian pedicle after ovariectomy. Circ. Res. 2001a;89:723–731. doi: 10.1161/hh2001.097870. [DOI] [PubMed] [Google Scholar]
- Patan S, Tanda S, Roberge S, Jones RC, Jain RK, Munn LL. Vascular morphogenesis and remodeling in a human tumor xenograft: blood vessel formation and growth after ovariectomy and tumor implantation. Circ. Res. 2001b;89:732–739. doi: 10.1161/hh2001.097872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Jung YD, et al. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15:1239–1241. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- Rhodin JA, Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J. Submicrosc. Cytol. Pathol. 1989;21:1–34. [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57:765–772. [PubMed] [Google Scholar]
- Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Wartenberg M, Donmez F, Ling FC, Acker H, Hescheler J, Sauer H. Tumor-induced angiogenesis studied in confrontation cultures of multicellular tumor spheroids and embryoid bodies grown from pluripotent embryonic stem cells. FASEB J. 2001;15:995–1005. doi: 10.1096/fj.00-0350com. 10.1096/fj.00-0350com. [DOI] [PubMed] [Google Scholar]
- Wilting J, Birkenhager R, Eichmann A, et al. VEGF121 induces proliferation of vascular endothelial cells and expression of flk-1 without affecting lymphatic vessels of chorioallantoic membrane. Dev. Biol. 1996;176:76–85. doi: 10.1006/dbio.1996.9993. 10.1006/dbio.1996.9993. [DOI] [PubMed] [Google Scholar]
- Yuan A, Yu CJ, Kuo SH, et al. Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J. Clin. Oncol. 2001;19:432–441. doi: 10.1200/JCO.2001.19.2.432. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Scott PA, Morbidelli L, et al. The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo. Br. J. Cancer. 2000;83:63–68. doi: 10.1054/bjoc.2000.1279. 10.1054/bjoc.2000.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Egginton S, Hudlicka O, Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res. 1998;293:293–303. doi: 10.1007/s004410051121. 10.1007/s004410051121. [DOI] [PubMed] [Google Scholar]