Abstract
Nitric oxide (NO) plays an important role in gastric mucosal injury in the human stomach. Exposure to excessive NO leads to apoptosis; however, the mechanism remains largely unknown in gastric epithelial cells. The apoptotic process is modulated by energy states in cells. This study investigated molecular mechanisms of NO-induced apoptosis in gastric epithelial cells and influence of high glucose on those mechanisms. Normal rat gastric mucosal epithelial (RGM-1) cells were cultured in media containing either 1000 (low) or 4500 mg/l (high) of D-glucose. When the cells were incubated with a chemical NO donor NOC18, apoptosis was induced in a dose-dependent manner. Intracellular adenosine triphosphate (ATP) levels significantly increased in the cells cultured with high glucose in comparison with the low-glucose condition. The cells with high ATP levels were more resistant to NO-induced apoptosis than the cells with low ATP levels. NO-induced apoptosis was followed by mitochondrial depolarization, upregulation of Bax protein, cytochrome C release from mitochondria to the cytosol and subsequent caspases activation. These results suggest that NO inhibition of mitochondrial respiratory system and acute ATP depletion initiate apoptotic signalling in gastric epithelial cells. High glucose may prevent NO-induced apoptosis by leading to high levels of intracellular ATP or other metabolic changes in this cell line.
Keywords: apoptosis, ATP, glucose, mitochondria, nitric oxide, stomach
Nitric oxide (NO) is a gaseous radical that functions as a potent bioactive mediator on various tissues in vivo (McDonald & Murad 1996; Beckman & Koppenol 1996). In the stomach, NO plays an important role in various gastric mucosal functions, such as acid secretion, blood flow, motility, mucosal defence and so on (Guth 1992; Suzuki et al. 1997; Elliott & Wallace 1998; Fischer et al. 1999).
Many studies have demonstrated that the excessive production of NO via inducible NO synthase (iNOS) and its derivatives participate in the pathogenesis of human gastric mucosal injury induced by Helicobacter pylori infection (Mannick et al. 1996; Fu et al. 1999; Slomiany et al. 1998). Recently, it has been reported that high concentrations of NO are generated at the gastroesophageal junction and cardia through the chemical reaction of salivary nitrite reduced by the action of ascorbic acid and acidic gastric juice and may contribute to the high incidence of mutagenesis and neoplasia in this area (Iijima et al. 2002). This suggests that the cytotoxic effects of NO on the gastric mucosal cells play an important role in the formation of various pathological features in the human stomach. However, the mechanism of gastric mucosal injury induced by NO remains largely unknown.
Exposure to an excess of NO has been reported to induce apoptotic cell death in a variety of cell-types, i.e. macrophages (Ushmorov et al. 1999), neurones (Uehara et al. 1999) and pancreatic β-cells (Okuno et al. 1998). NO-induced apoptosis is accompanied by nuclear DNA damage, excessive activation of poly-(adenosine diphosphate-ribose)-polymerase or disarrangement of calcium metabolism (Burney et al. 1999; Murphy 1999). In addition, the cytotoxic effects of NO cause inhibition of the mitochondrial respiration and result in acute depletion of adenosine triphosphate (ATP) (Almeida et al. 2001; Brown & Borutaite 2002). NO inhibition of mitochondrial respiration accelerates glycolysis in astrocytes but not in neurones, and this difference is reported to affect the sensitivity to NO-induced apoptosis in both cells (Almeida et al. 2001). In contrast, this can induce necrosis to neural cells instead of apoptosis, if glycolysis is also inhibited or is insufficient to compensate ATP depletion (Brown & Borutaite 2002). These findings suggest that intracellular energy states including glucose metabolism may affect cellular responses to NO cytotoxicity.
This study investigates the molecular mechanism of NO-induced apoptosis and the influence of glucose metabolism on energy states and NO-induced apoptosis by using the normal rat gastric mucosal RGM-1 cells (Kobayashi et al. 1996) incubated in either low- or high-glucose–Dulbecco's modified Eagle's (DME) media and, a pure NO donor, NOC-18 (Mooradian et al. 1995).
Materials and methods
Cell culture
RGM-1 cell line (rat gastric mucosal cell first, RIKEN Cell Bank RCB0876) (Kobayashi et al. 1996) was cultured in DME media containing D-glucose at either concentrations of 1000 mg/l (low-glucose–DME medium) or 4500 mg/l (high-glucose–DME medium) (Gibco, Rockville, MD, USA), respectively. The media were supplemented with 20% (v/v) fetal bovine serum (FBS). After the cells grew to approximately 70–80% confluence in these media in 5% CO2 at 37 °C, the cells were incubated in a freshly prepared serum-free low-glucose–DME medium containing various concentrations of NOC-18 (Dojindo, Kumamoto, Japan) for 24 h in 5% CO2 at 37 °C.
Assessment of intracellular ATP level
The intracellular ATP level was measured by a commercially available ATP assay system (LL-100; Toyo Ink, Tokyo, Japan) according to the manufacturer's instructions. The system can quantify ATP by measuring the chemical luminescence emitted by the luciferase reaction in the presence of ATP and luciferine. The amounts of chemical luminescence emitted for 10 s due to the reaction were measured with a luminometer Lumat LB 9507 (EG & G Berthold, Bad Wildbad, Germany). The amount of ATP was determined from a standard curve based on given ATP solutions. For comparison, results are expressed as the mean percentage ratios of ATP contents per 104 cells in the cells incubated in the high-glucose medium to the cells incubated in the low-glucose medium.
Assessment of cell viability
Cell viability after NOC-18 treatment was assessed with a colourimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Twentyman 1987) and a Trypan-blue-dye exclusion test (Philips 1973). Results are expressed as the mean percentage ratios of the absorbance of the MTT reagent in the cells treated with NOC-18 to the untreated cells or the percentage mean ratios of the number of living cells after the treatment to the untreated cells, respectively.
Detection of apoptotic cell death
The characteristics of apoptosis were examined by chromatin staining with Hoechst 33258-dye (Wako Pure Chemical, Osaka, Japan) and by TdT-mediated dUTP-biotin nick-end labelling (TUNEL) (Gavrieli et al. 1992). After NOC-18 treatment, the cells were collected, fixed in 3% paraformaldehyde and, then, spread on glass slides in preparation for Hoechst 33258-dye staining and TUNEL. TUNEL was performed with an Apoptosis in situ Detection Kit (Wako Pure Chemical, Osaka, Japan) according to the manufacturer's instructions.
Assessment of mitochondrial membrane potential
The mitochondrial membrane potential (ΔΨ) as an indicator of mitochondrial function was assayed by a cationic dye JC-1 Mitochondrial Potential Sensor (Molecular Probes, Eugene, OR, USA), which accumulates in mitochondria and shows a fluorescence emission shift from green (525 nm) to red (590 nm) in a potential-dependent manner. The red/green fluorescence ratio (Fr/g) of the dye is dependent on the ΔΨ, and a decrease indicates mitochondrial depolarization. After treatment, the JC-1 dye was applied to RGM-1 cells and the intensity of fluorescence at 538 (green) and 590 nm (red) (excitation wave-length: 485 and 544 nm) was measured with a fluorometer Fluoroskan Ascent (Labsystems, Helsinki, Finland) according to the manufacturer's instructions. Results are expressed as the mean ratios of Fr/g in the subjective cells to the cells growing in the low-glucose–DME medium.
Western blot analysis for Bcl-2, Bax and cytochrome C
Fifty micrograms each of the protein sample was fractionated by 12.5% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). After blocking nonspecific bindings, the membranes were probed with anti-Bax (1 : 500, P-19, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-Bcl-2 (1 : 500, ΔC21, Santa Cruz Biotechnology) antibody, followed by the application of appropriate sets of horseradish peroxidase-conjugated secondary antibodies (Amersham Pharmacia, Buckinghamshire, UK). An enhanced chemiluminescence detection system (Amersham Pharmacia) was used for the protein detection according to the manufacturer's instructions.
The release of cytochrome C from the mitochondria to the cytosol in the process of apoptosis was assessed by Western blot analysis using protein samples extracted individually from either the fraction of the mitochondria or that of the cytosol, which were collected separately from the cells according to the method of Yang et al. (Yang et al. 1997). Twenty-five micrograms each of the protein sample was used for the Western blotting. An anti-cytochrome C antibody (7H8.2C12, BD PharMingen, San Diego, CA, USA) was used as the primary antibody at a dilution of 1 : 1000.
Assessment of caspase-3, -8 and -9 activity
The activity of caspase-3, -8 and -9 were measured with a Colourimetric Protease Assay Kit (MBL, Nagoya, Japan). This system is able to determine respective caspase activity by the colourimetric quantification of p-nitroanilide (pNA), which is liberated from pNA-labelled substrates by the proteolytic action of the active form of the caspase. Caspase activity was expressed as fold increments of the photoabsorbance of pNA at 405 nm in the treated cells to the control.
Statistical analysis
Results are expressed as the mean ± SE of at least three repetitions. The data were analysed by Fisher's protected least squares difference test. A difference with a P-value <0.05 was considered statistically significant.
Results
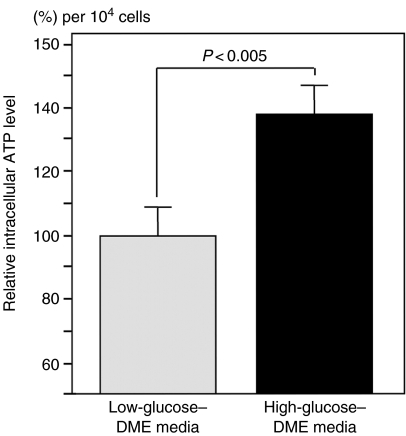
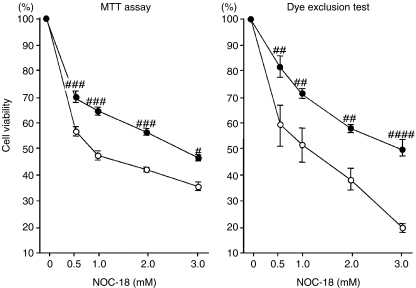
RGM-1 cells were cultured in either low-glucose– or high-glucose–DME media after reaching 70–80% confluence. The intracellular ATP level in the cells incubated in the low-glucose medium was 0.38 ± 0.15 pmol/104 cells and significantly increased in the cells growing in the high-glucose–DME medium, compared with the cells growing in the low-glucose–DME medium (P < 0.005; Figure 1). These cells were exposed to NOC-18 at serial concentrations from 0 to 3.0 mmol/l for 24 h and, thereafter, were assessed by MTT assay and Trypan-blue-dye exclusion test. Cell viability in both sets of cells was reduced by treatment with NOC-18 in dose-dependent manner; however, both assessments showed significantly higher values in the cells incubated in the high-glucose medium than those in the cells incubated in the low-glucose medium (MTT assay; NOC-18 from 0.5 to 2.0 mmol/l, P < 0.001; NOC-18 at 3.0 mmol/L, P < 0.01; Trypan-blue-dye exclusion test; NOC-18 from 0.5 to 2.0 mmol/l, P < 0.005; NOC-18 at 3.0 mmol/l, P < 0.0001; Figure 2).
Figure 1.
Intracellular adenosine triphosphate (ATP) levels in the cells cultured in low-glucose– or high-glucose–DME media. Intracellular ATP levels were assessed by measuring amounts of the chemical luminescence emitted by the liciferine/luciferase reaction in the presence of sample ATP. Results are expressed as the mean percentage ratios of ATP contents per 104 cells in the cells incubated in the high-glucose medium to the cells incubated in the low-glucose medium. Intracellular ATP levels significantly increased in the cells under the high-glucose condition (dotted column) in comparison with the cells under the low-glucose condition (black column, P < 0.005).
Figure 2.
Changes in cell viability after treatment with NOC-18. Normal rat gastric mucosal (RGM-1) cells incubated in either low- or high-glucose–DME media were treated with NOC-18 at the serial concentrations of 0, 0.5, 1.0, 2.0 and 3.0 mmol/l for 24 h. Cell viability was assessed by MTT assay and Trypan-blue-dye exclusion test. Black circles represent the cells in the high-glucose medium and white circles the low-glucose medium. Cell viability decreased in both sets of cells by treatment with NOC-18 dose-dependently; however, the values in the cells incubated in the high-glucose medium were significantly higher than those in the cells incubated in the low-glucose medium. Low glucose vs. high glucose at the same concentration of NOC-18. #, P < 0.01; ##, P < 0.005; ###, P < 0.0001.
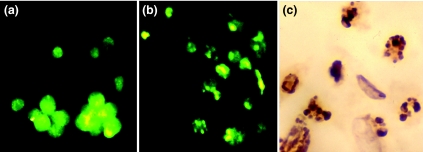
The cells killed by NOC-18 collected from the culture media showed morphologically characteristic features of apoptosis, such as chromatin condensation and nuclear fragmentation, in the fluorescence microscopic images of Hoechst 33258-dye staining (Figure 3a, b), and they also reacted positively to TUNEL staining (Figure 3c).
Figure 3.
Representative photographs of rat gastric mucosal (RGM-1) cells after treatment with NOC-18 using Hoechst 33258-dye staining and TdT-mediated dUTP-biotin nick-end labelling (TUNEL) (×200).(a) The untreated cells showed morphologically intact nuclei in a fluorescence microscopic image of Hoechst 33258-dye staining.(b) The cells treated with NOC-18 showed chromosomal condensation and nuclear fragmentation, which were characteristic of apoptosis.(c) The treated cells also reacted positively to TUNEL staining, whose brown reactants were detected only in the fragmented nuclei but not in the intact nuclei.
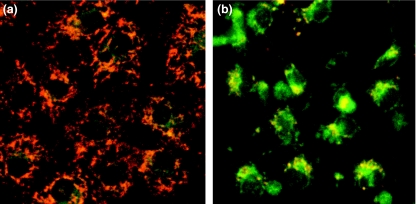
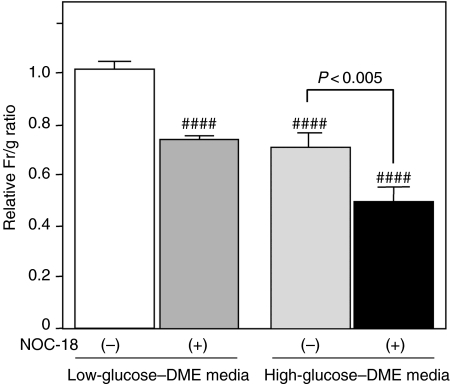
Changes in ΔΨ after treatment with NOC-18 were analysed using a cationic JC-1 dye. In synchronized images of green (538 nm) and red (590 nm) fluorescence, the untreated cells showed granular accumulations of the JC-1 dye, emitting red fluorescence, in the mitochondria; however, the dye in the cells treated with NOC-18 was diffusely extended in the cytosol, emitting green fluorescence (Figure 4a, b). Changes in ΔΨ were quantified as the relative ratios of the Fr/g-values in the cells subjected to NOC-18 treatment to the cells growing in the low-glucose–DME medium without NOC-18 treatment. The ratios significantly decreased in the cells treated with NOC-18 under the low-glucose condition and the cells incubated under the high-glucose condition, regardless treatment with NOC-18 (P < 0.0001), although treatment with NOC-18 significantly promoted the reduction of relative Fr/g ratios in the cells under the high-glucose condition (P < 0.005, Figure 5).
Figure 4.
Representative photographs of rat gastric mucosal (RGM-1) cells after treatment with NOC-18 by JC-1-dye staining. Synchronized images of green (538 nm) and red (590 nm) fluorescence of JC-1-dye staining;(a) the untreated cells and (b) the cells treated with NOC-18. Granular accumulations of the JC-1 dye, emitting red fluorescence in mitochondria, were detected in the untreated cells, but the dye in the cells treated with NOC-18 was diffusely extended in the cytosol, emitting green fluorescence, indicating mitochondrial depolarization in the latter.
Figure 5.
Changes in mitochondrial transmembrane potential after treatment with NOC-18. Changes in the membrane potential (ΔΨ) are quantified as relative ratios of Fr/g-values. White column, the cells incubated with low glucose without NOC-18 treatment; grey column, the cells treated with NOC-18 under low-glucose condition; dotted column, the cells incubated with the high-glucose medium without NOC-18 treatment; black column, the cells treated with NOC-18 under high-glucose condition. The ratios significantly decreased in the cells treated with NOC-18 under low-glucose condition and the cells incubated with the high-glucose medium, regardless treatment with NOC-18 (####, P < 0.0001), although treatment with NOC-18 promoted mitochondrial depolarization in the cells under high-glucose condition (P < 0.005).
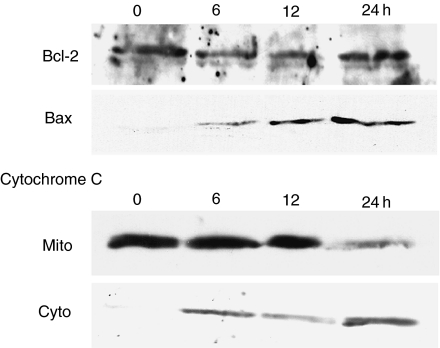
Temporal changes in the expression of Bcl-2 and Bax proteins in the cells incubated in the low-glucose medium were also assessed by Western blotting. The Bax protein, which was observed as a band with approximately 21 kDa, was not detected in the control (time zero). But its expression appeared after 6 h of treatment with NOC-18 and increased gradually (Figure 6). The protein expression of Bcl-2, which was fractioned at 26 kDa, did not show apparent change after treatment with NOC-18, compared with the control (Figure 6).
Figure 6.
Temporal changes in the expression of Bcl-2- and Bax-proteins and the release of cytochrome C from mitochondria to the cytosol. Temporal changes in the expression of Bcl-2- and Bax-proteins and the release of cytochrome C from mitochondria to the cytosol in the cells treated with NOC-18, under low-glucose condition, were analysed by Western blotting. Bax protein was not detected in the control (time zero), but its expression increased gradually after treatment with NOC-18 (the first rank). The protein expression of Bcl-2 did not show any change compared with the control (the second rank). Western blotting for cytochrome C was performed using individual protein samples from either mitochondrial fractions (Mito, the third rank) or cytosolic fractions (Cyto, the fourth rank). In the control, cytochrome C was detected only in the mitochondrial fraction. After treatment with NOC-18, it weakly appeared in the cytosolic fraction, 6 h later, and was gradually increasing. The density of cytochrome C immunoreactants was ultimately inverted between both fractions, 24 h later.
Release of cytochrome C from the mitochondria to the cytosol was assessed by Western blotting using protein samples that were extracted individually from either the mitochondrial fraction or the cytosolic fraction in the cells incubated with the low glucose. In the control, cytochrome C with a molecular size of 15 kDa was present in the mitochondrial fraction but was barely observed in the cytosolic fraction (Figure 6). After treatment with NOC-18, it weakly appeared in the cytosolic fraction, 6 h later, and the intensity of the band was ultimately inverted between the mitochondrial and the cytosolic fraction, 24 h later (Figure 6).
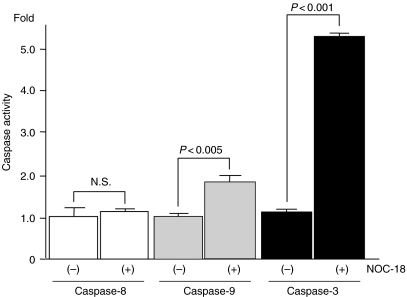
Changes in intracellular activity of caspase-3, -8 and -9 in the cells incubated in the low-glucose medium, after treatment with NOC-18, were examined using commercially available colourimetric assay kits. Both the activity of caspase-3 and -9 significantly increased in the cells treated with NOC-18 (caspase-3, P < 0.0001; caspase-9, P < 0.005; Figure 7); however, there was no difference in the activity of caspase-8 between the treated cells and the untreated cells (Figure 7).
Figure 7.
Changes in the intracellular activity of caspase-3, -8 and -9. Changes in the intracellular activity of caspase-3, -8 and -9 in the cells treated with NOC-18, under low-glucose condition, were expressed as their fold increments in the cells treated with NOC-18 to the untreated cells. Both the activity of caspase-3 and -9 significantly increased in the cells treated with NOC-18 (caspase-3, P < 0.0001; caspase-9, P < 0.005); however, there was no difference in the activity of caspase-8 between the treated cells and the untreated cells.
Discussion
NO functions as a potent bioactive mediator in the stomach (Guth 1992; Suzuki et al. 1997; Elliott & Wallace 1998; Fischer et al. 1999) and its cytotoxic effects are supposed to play a critical role in the formation of various pathological features in human gastric mucosa (Mannick et al. 1996; Fu et al. 1999; Slomiany et al. 1998). However, the mechanism of NO-induced apoptosis in gastric epithelial cells remains largely unknown. In addition, recent studies have shown that intracellular metabolic states of glucose may affect responses to NO cytotoxicity (Almeida et al. 2001; Brown & Borutaite 2002). Therefore, this study also investigated the influence of differential concentrations of extracellular glucose on NO-induced apoptosis in RGM-1 cells.
Treatment with a chemical, NO donor NOC-18, induces apoptotic cell death in RGM-1 cells dose-dependently, consistent with other types of cell (Ushmorov et al. 1999; Uehara et al. 1999; Okuno et al. 1998; Burney et al. 1999; Murphy 1999; Almeida et al. 2001; Brown & Borutaite 2002). The cells incubated under the high-glucose condition possessed high levels of intracellular ATP and were more resistant to the induction of apoptosis by treatment with NOC-18 than the cells incubated under the low-glucose condition and with low ATP levels. High concentration of glucose is also reported to promote cell survival against spontaneous and Fas-mediated apoptosis in neutrophils and pancreatic β-cells (Healy et al. 2002; Srinivasan et al. 2002). Our results were compatible with these reports.
NO cytotoxicity causes mitochondrial dysfunction and leads to reduction of mitochondrial membrane potential (δσ) (Almeida et al. 2001; Brown & Borutaite 2002). In the present study, changes in the δσ were quantitatively assessed by comparison with relative Fr/g ratios of a cationic dye JC-1 Mitochondrial Potential Sensor. Consistent with previous reports, treatment with NOC-18 caused mitochondrial depolarization in RGM-1 cells, regardless of glucose concentrations. These results suggest that high concentrations of extracellular glucose increases in intracellular ATP levels and may affect responses to the stimulation of NO-induced apoptosis in gastric epithelial cells. Brown et al. have shown that NO inhibition of mitochondrial respiration causes acute ATP depletion, but compensation by acceleration of glycolysis delays the induction of apoptosis in astrocytes (Brown & Borutaite 2002). In contrast, neurones were more sensitive to NO cytotoxicity, because they do not possess such compensatory system for ATP depletion by glycolysis (Brown & Borutaite 2002). These data are compatible and supportive of our hypothesis. An increase in intracellular ATP through glycolysis due to the incubation in the high-glucose medium may strengthen the protective ability against NO cytotoxicity in gastric epithelial cells.
High-glucose condition is also reported to activate a phosphatidylinositol (PI) 3-kinase/Akt-signal pathway and cause mitochondrial depolarization and calcium influx, which can work for cell survival of pancreatic β-cells (Srinivasan et al. 2002). Interestingly, the reduction of ΔΨ was also observed in RGM-1 cells incubated in the high-glucose condition unless the cells were treated with NOC-18. This suggests that mitochondrial depolarization and calcium influx as a consequence of the activation of PI3-kinase/Akt-signal pathway may be also involved in preventive effects of high glucose against NO-induced apoptosis in RGM-1 cells. Moreover, recently, a pro-apoptotic Bcl-2 family member BAD and glucokinase are reported to form a mitochondrial functional complex that integrates glycolysis and apoptosis (Danial et al. 2003). This may participate in the glucose-dependent modulation of susceptibility to NO-induced apoptosis in RGM-1 cells.
The signal transduction system of apoptosis induced by NO in gastric epithelial cells has not been comprehensively studied. NO-induced apoptosis in RGM-1 cells was followed by an increased expression of Bax protein, release of cytochrome C from the mitochondria to the cytosol and subsequent activation of caspase-9 and -3 but not caspase-8. These results are consistent with those of previous reports on NO-induced apoptosis using Jurkat leukaemic cells (Ushmorov et al. 1999) and human neuroblastoma SH-SY5Y cells (Uehara et al. 1999). The upregulation of Bax facilitates the release of cytochrome C from the mitochondria to the cytosol, which activates the cascade of caspase-9 and -3 together with Apaf-1 in the cytosol (Liu et al. 1996; Zou et al. 1997). This process is ordinarily followed by apoptotic cell death. However, Okuno et al. (Okuno et al. 1998) reported that the death of PC12 and HeLa cells induced by exogenous NO was enhanced by Bax expression in a caspase-independent manner, showing features of both apoptosis and necrosis with chromatin condensation, nuclear compaction and mitochondrial swelling.
Exposure to an excess of NO leads to apoptosis, necrosis or cell death with combined features as shown in previous reports (Ushmorov et al. 1999; Uehara et al. 1999; Okuno et al. 1998). In contrast, NO inversely acts as an anti-apoptotic substance under some conditions, leading to the inactivation or inhibition of caspase processing by S-nitrosation (Li et al. 1997; Rossig et al. 1998). Such a diversity of responses to NO, which is thought to depend on cell type, the state of energy production, the condition of NO exposure and the severity or context of damage confuses the role of NO in apoptosis induction. Further studies are required to fully elucidate the role of NO in apoptosis induction.
Conclusion
This study investigated the molecular mechanism of cytotoxicity of NO using a pure NO donor, NOC-18, and RGM-1 cells. The results suggest that an excess of NO induces apoptosis in gastric epithelial cells through the disruption of energy production in mitochondria, upregulation of Bax protein, cytochrome C release from mitochondria to the cytosol and subsequent activation of caspase-9 and -3. High glucose may prevent NO-induced apoptosis by leading to high levels of intracellular ATP or other metabolic changes in this cell line.
References
- Almeida A, Almeida J, Bolanos JP, Moncada S. Different responses of astrocytes and neurones to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc. Natl. Acad. Sci. U. S. A. 2001;98(26):15294–15299. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic. Biol. Med. 2002;33(11):1440–1450. doi: 10.1016/s0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res. 1999;424(1–2):37–49. doi: 10.1016/s0027-5107(99)00006-8. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424(6951):952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Elliott SN, Wallace JL. Nitric oxide: a regulator of mucosal defence and injury. J. Gastroenterol. 1998;33(6):792–803. doi: 10.1007/s005350050178. [DOI] [PubMed] [Google Scholar]
- Fischer H, Becker JC, Boknik P, et al. Expression of constitutive nitric oxide synthase in rat and human gastrointestinal tract. Biochim. Biophys. Acta. 1999;1450(3):414–422. doi: 10.1016/s0167-4889(99)00049-x. [DOI] [PubMed] [Google Scholar]
- Fu S, Ramanujam KS, Wong A, et al. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116(6):1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- Guth PH. Current concepts in gastric microcirculatory pathophysiology. Yale J. Biol. Med. 1992;65(6):677–688. [PMC free article] [PubMed] [Google Scholar]
- Healy DA, Watson RW, Newsholme P. Glucose, but not glutamine, protects against spontaneous and anti-Fas antibody-induced apoptosis in human neutrophils. Clin. Sci. (Lond). 2002;103(2):179–189. doi: 10.1042/cs1030179. [DOI] [PubMed] [Google Scholar]
- Iijima K, Henry E, Moriya A, Wirz A, Kelman AW, McColl KE. Dietary nitrate generates potentially mutagenic concentrations of nitric oxide at the gastroesophageal junction. Gastroenterology. 2002;122(5):1248–1257. doi: 10.1053/gast.2002.32963. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Kawano S, Tsuji S, et al. RGM1, a cell line derived from normal gastric mucosa of rat [letter] In Vitro Cell Dev. Biol. Anim. 1996;32(5):259–261. doi: 10.1007/BF02723056. [DOI] [PubMed] [Google Scholar]
- Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem. Biophys. Res. Commun. 1997;240(2):419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- Mannick EE, Bravo LE, Zarama G, et al. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- McDonald LJ, Murad F. Nitric oxide and cyclic GMP signaling. Proc. Soc. Exp. Biol. Med. 1996;211(1):1–6. doi: 10.3181/00379727-211-43950a. [DOI] [PubMed] [Google Scholar]
- Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J. Cardiovasc. Pharmacol. 1995;25(4):674–678. [PubMed] [Google Scholar]
- Murphy MP. Nitric oxide and cell death. Biochim. Biophys. Acta. 1999;1411(2–3):401–414. doi: 10.1016/s0005-2728(99)00029-8. [DOI] [PubMed] [Google Scholar]
- Okuno S, Shimizu S, Ito T, et al. Bcl-2 prevents caspase-independent cell death. J. Biol. Chem. 1998;273(51):34272–34277. doi: 10.1074/jbc.273.51.34272. [DOI] [PubMed] [Google Scholar]
- Philips HI. Dye exclusion tests for cell viability. In: Kruse PF, Patterson MK, editors. Tissue Culture, Method and Applications. New York: Academic Press; 1973. pp. 407–408. [Google Scholar]
- Rossig L, Fichtlscherer B, Breitschopf K, et al. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J. Biol. Chem. 1999;274(11):6823–6826. doi: 10.1074/jbc.274.11.6823. [DOI] [PubMed] [Google Scholar]
- Slomiany BL, Piotrowski J, Slomiany A. Induction of caspase-3 and nitric oxide synthase-2 during gastric mucosal inflammatory reaction to Helicobacter pylori lipopolysaccharide. Biochem. Mol Biol. Int. 1998;46(5):1063–1070. doi: 10.1080/15216549800204612. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Bernal-Mizrachi E, Ohsugi M, Permutt MA. Glucose promotes pancreatic islet beta-cell survival through a PI 3-kinase/Akt-signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2002;283(4):E784–E793. doi: 10.1152/ajpendo.00177.2002. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Shimosegawa T, Satoh A, et al. Gastric mucosal blood flow response to stress in streptozotocin diabetic rats: regulatory role of nitric oxide. J. Gastroenterol. 1997;32(6):726–733. doi: 10.1007/BF02936947. [DOI] [PubMed] [Google Scholar]
- Twentyman PR. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer. 1987;56:279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Kikuchi Y, Nomura Y. Caspase activation accompanying cytochrome C release from mitochondria is possibly involved in nitric oxide-induced neuronal apoptosis in SH-SY5Y cells. J. Neurochem. 1999;72(1):196–205. doi: 10.1046/j.1471-4159.1999.0720196.x. [DOI] [PubMed] [Google Scholar]
- Ushmorov A, Ratter F, Lehmann V, Droge W, Schirrmacher V, Umansky V. Nitric-oxide-induced apoptosis in human leukemic lines requires mitochondrial lipid degradation and cytochrome C release. Blood. 1999;93(7):2342–2352. [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome C from mitochondria blocked. Science. 1997;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome C-dependent activation of caspase-3. Cell. 1997;90(3):405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]