Abstract
Mortality caused by septic shock in experimental animals is reduced by thalidomide, an inhibitor of tumour necrosis factor α. Another drug that could act on the pathopysiological mechanisms of septic shock is pentoxifylline, an inhibitor of platelet aggregation that increases the flexibility of the erythrocyte membrane and has fibrinolytic activity. We studied the effect of pentoxifylline alone and combined with thalidomide in septic shock; 97 NIH mice were injected with lipopolysaccharides of Salmonella abortus equi and D galactosamine. Animals were separated in 4 groups; group A (n = 20) was used as control, group B (n = 15) received thalidomide 50 mg/kg, group C (n = 20) received pentoxifylline 40 mg/kg, and group D (n = 15) received thalidomide plus pentoxifylline. Mortality was recorded every hour. Additionally, 5 animals from each group were sacrificed 8 h after the induction of septic shock for histological analysis of heart, lung, brain, kidney, small intestine, adrenal glands and liver. Microscopic findings were rated as absent, mild, moderate and severe damage. In control animals histological analysis showed intense haemorrhage and necrosis in all organs studied. When compared with controls, treatment with pentoxifylline plus thalidomide reduced mortality (P < 0.03). The tissue damage was less severe in animals from the groups that received pentoxifylline or pentoxifylline plus thalidomide (P < 0.05). Pentoxifylline seems to potentiate the beneficial effects of thalidomide, reducing mortality and attenuating the pathological changes produced by septic shock.
Keywords: septic shock, pentoxifylline, thalidomide, histopathology
Septic shock is characterized by hypotension and organic dysfunction (Hardawat 1996), as a consequence of a widespread immune response induced by bacterial lipopolysaccharides. Apparently, the most important mediator is tumour necrosis factor α (TNFα) (Lesslauer et al. 1991; Mohler et al. 1994), a cytokine that stimulates phospholipase A2 (Danner 1991; Vadas & Pruzanski 1993) and participates in the magnification of the response mediated by the release of other cytokines and active substances such as the proinflammatory interleukines (IL) 1,6 and 8, kalycreine, factor XII, platelet activator factor and nitric oxide.
Mortality caused by septic shock is high, treatments are frequently ineffective (American College of Chest Physicans/Society of Critical Care Medicine Consensus Conference Committee 1992). Thalidomide, an inhibitor of the synthesis of TNFα has been used in the treatment of leprosy lupus, AIDS, billiary cirrhosis, experimental brain tumours, and bone marrow transplant (Randall 1990; Koch 1994; Palencia et al. 1998). In experimental models of septic shock, previous administration of thalidomide reduced mortality (Schmidt et al. 1996). Pentoxifylline, a dimethylxanthine with fibrinolytic activity an inhibit of platelet aggregation increases the flexibility of erythrocytes thus improving the tissue perfusion in patients with arterial stenosis (Frampton & Brogden 1995); it also decreases the inflammation associated with experimental bacterial meningitis (Saez-Llorens et al. 1994; Ostrosky et al. 1996). Our aim was to asses the effect of pentoxifylline alone and combined with thalidomide in a murine model of septic shock.
Materials and methods
Pharmacological compounds
Thalidomide was synthesized in the Orphan Drug laboratory at the Universidad Autónoma Metropolitana (Mexico City), the isomer used was the teratogenically active one. Pentoxifylline was the commercially available compound (Hoeschst-Roussel, Mexico City).
Induction of septic shock
97 male NIH mice aged 8–10 weeks, were injected intraperitoneally with 10 μg of lipopolysaccharides (LPS) from Salmonella abortus equi (Sigma-Aldrich Chemical Co., Mexico City) diluted in 0.1 ml of PBS simultaneously, 18 mg of d-galactosamine (D-GalN, from Sigma, St Louis, MO) diluted in 0.1 ml of PBS were injected in the another side of the peritoneal cavity (Lehmann et al. 1987). Mortality was monitored every hour over a period of 30 h. No further mortality was observed after this time.
Drug treatments
The treatment schedule was initiated immediately after induction of septic shock. 70 animals were randomly assigned to four groups. Animals from group A (n = 20) were orally treated with nonpyrogenic saline solution (positive controls); group B (n = 20) received 40 mg/kg body weight of pentoxifylline by intraperitoneal injection; group C (n = 15) were orally treated, through a catheter, with thalidomide at 50 mg/kg; group D (n = 15) received thalidomide plus pentoxifylline in the same doses as animals from groups B and C.
Histopathological evaluation
Additional to the experiments of survival, five healthy animals (negative controls), 10 mice from the positive control group, and five animals from each treatment group were sacrificed by cervical dislocation 8 h after induction of septic shock and immediately perfused with a solution of 10% formaline in saline solution. The brain, lungs, kidneys, adrenals glands, heart, and liver were dissected and fixed in the same solution for 2 weeks. Afterwards, the specimens were dehydrated, embedded in paraffin, sectioned and stained with haematoxilin and eosin by the conventional histological procedures. All tissue samples were assessed blindly by the same pathologist (D.R.), who graded the main alterations, necrosis and haemorrhage, observed on every organ according to the following scale: absent (0), mild (1), moderate (2) and severe (3).
Statistical analysis
Survival time and histophatological damage were compared between groups by the Kruskal–Wallis analysis of variance and the Mann-Witney U-test; mortality was compared by the Fisher's exact test. α level was set at P < 0.05.
Results
The model of septic shock used in these experiments induced a high mortality of 77% in untreated mice; mortality in animals treated with thalidomide or with pentoxifylline alone was similar. However, the group of animals treated with thalidomide plus pentoxifylline had a significant survival increase (P < 0.03) compared with controls or with animals treated with each drug alone (Table 1). Survival according to the Kaplan Meier plot showed significant differences of cummulative survival in animals treated with thalidomide/pentoxifylline when compared with controls or with animals treated either with thalidomide or with pentoxifylline alone (Figure 1). Histopathological analysis in controls with septic shock showed intense of haemorrhage and necrosis in all organs studied, particularly liver and kidney (Figure 2). Degree of damage was significantly minor in animals treated either with pentoxifylline or with pentoxifylline plus thalidomide (P < 0.05); no difference was found in animals treated with thalidomide alone (Figure 3).
Table 1.
Survival of mice with septic shock

* Kruskal − Wallis analysis of variance followed by multiple comparations of all treatment groups with the infected control by the Mann − Whitney U— test;
† P < 0.03 with Fisher's exact Test.
Figure 1.
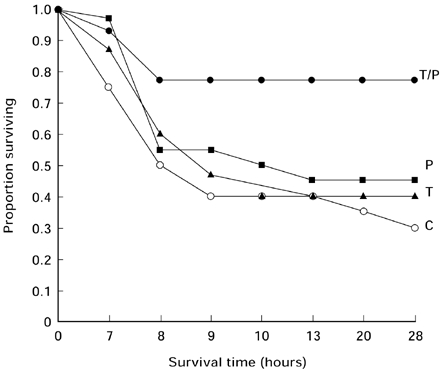
Survival data after induction of septic shock are shown as a Kaplan Meier plot, cummulative survival in animals treated with thalidomide/pentoxifylline was significantly greater than the other groups (P < 0.03). ○ controls; ▴ thalidomide; ▪ pentoxifylline; • thalidomide + pentoxifylline
Figure 2.
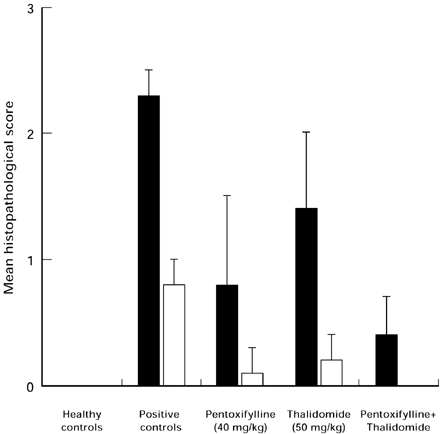
Mean results of histopathological findings in the liver (▪) and kidney (□) of healthy animals and in animals with septic shock treated with thalidomide and/or pentoxifylline. *P < 0.05 when compared with positive controls.
Figure 3.
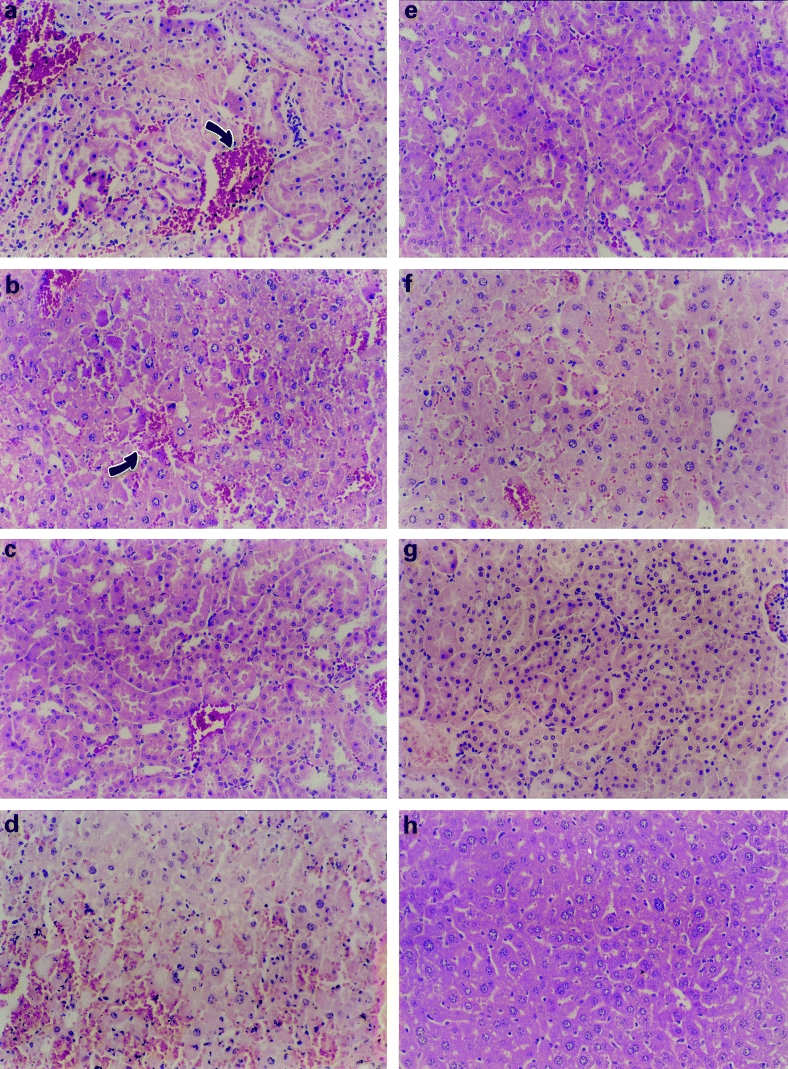
Microscopic appearance of kidney and liver of animals after septic shock. Positive controls show (a) in kidney severe tubular necrosis, desquamated cells in the lumen, haemorrhage and necrosis (arrow) and (b) in liver haemorrhage and disorganization into the parenchyma with degenerating hepatocytes (arrow). In animals treated with thalidomide (c) the kidney still shows tubular necrosis with hyperchromatic nuclei and haemorrhage and (d) the liver had eosinophilic cytoplasm, shrunken hepatocytes and haemorrhage. In animals treated with pentoxifylline the above mentioned abnormalities are minor (e) in kidney and (f) in liver. In animals treated with a combination of pentoxifylline/thalidomide the signs of damage caused by septic shock are notably attenuated (g and h). (HE, 200 ×)
Discussion
In humans, mortality from sepsis remains close to 50%, this unacceptable rate has stimulated interest in the search for pharmacological agents that might reduce morbility and mortality (Glauser et al. 1994). One option is the combination of drugs that interferes with the physiopathological mechanisms of immune response triggered by systemic sepsis. Our results show that the combination of pentoxifylline plus thalidomide has a protective effect on mortality and on tissue damage produced by septic shock in mice. This protective effect was particularly evident in the damage induced in liver and kidney, the most severely affected organs; protection seemed to be more conspicuous in the kidney than the liver. We found histophatological changes characteristic of severe hypoperfusion (Krausz & Cohen 1992), the microscopical examination reveals tubular necrosis, sinusoidal dilatation, congestion with necrosis that affects acinar zone 3 and 2. In contrast, administration of thalidomide alone had minor efficacy compared with administration of pentoxifylline or pentoxifylline plus thalidomide. Other reports have shown that thalidomide administered as pretreatment for septic shock increases survival from acute endotoxaemia, this contrasting finding may be due to the blockage of TNF when thalidomide is given before the induction of toxaemia (Schmidt et al. 1996). In this study, however, thalidomide was administered after the induction of septic shock, where it had only a moderate but nonsignificant protective effect. We chose to administer thalidomide not as a preventive drug but as treatment for septic shock after its mechanisms had already been incited. The compound used by us is in a crystal/powder form, virtually insoluble in aqueous, alcohol and oil medium; therefore, it only achieves adequate serum concentrations by oral administration. Serum levels are detectable by liquid chromatography within 1 h of oral administration and the peak levels are seen after 2–4 h. The serum half life is between 12 and 18 h (Eriksson et al. 1992a,b). These pharmacodynamic characteristics may diminish the interaction timing of thalidomide with peak levels of TNFα production during the course of septic shock, thus explaining its lack of effectiveness in our experiments. Other studies have found a favourable effect of pentoxifylline in the course of septic shock, particularly when combined with methylxantine (Jilg et al. 1996).
Pentoxifylline is a dimethylxantine known for fibrinolytic activity and its ability to inhibit platelet aggregation (Ambrus et al. 1995), it is widely used in humans for treatment of vascular diseases such as intermittent claudication (Hood et al. 1996). Other studies have found a favourable effect of pentoxifylline in the course of septic shock, particularly when combined with methylxantine (Jilg et al. 1996). Pentoxifylline regulates the release of IL-1β, IL-6, IL-8 and TNFα in blood by mononuclear cells (Neuner et al. 1994), it also suppresses Interleukin-2 by inhibition of endogenous TNFα (Jewett & Bonavida 1994), additionally pentoxifylline reduces adhesion molecule expression (Mc Bride et al. 1996). These effects on cytokines could interfere with complex immune mechanisms incited by toxaemia, thus explaining the decrease in mortality and reduction of histopatological changes obtained when the drug was administrated jointly with thalidomide perhaps as a consequence of mutual pharmacological potentiation.
Acknowledgments
We thank Camilo Rios Ph.D. for his valuable help in the statistical analysis and Dr Jaime Kravzov Universidad Autonoma Metropolitana for the gift of thalidomide. This work was partly supported by The National Council of Science and Tecnology (CONACYT) grant No. L0001-M9608.
References
- Ambrus JL, Stadler S, Kulaylat M. Hemorreologic effects of metabolites of Pentoxifylline. J. Med. 1995;26:65–75. [PubMed] [Google Scholar]
- American College of Chest Physicans/Society of Critical Care Medicine Consensus Conference Committee. Definitions for sepsis and organ failure and guidelines for use of innovative therapies in sepsis. Crit. Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- Danner RL. Endotoxemia in human septic shock. Chest. 1991;99:169. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- Eriksson T, Bjorkman S, Fyge A, Ekberg H. Determination of thalidomide in plasma and blood by high-performance liquid chromatography: avoiding hydrolytic degradation. J. Chromatogr. 1992a;582:211–216. doi: 10.1016/0378-4347(92)80321-g. [DOI] [PubMed] [Google Scholar]
- Eriksson T, Riesbeck K, Ostraat O, Ekberg H, Bjorkman S. Drug exposure and flow cytometry analyses in a thalidomide schedule that prolongs rat cardiac graft survival. Transplant. Proc. 1992b;24:2560–2561. [PubMed] [Google Scholar]
- Frampton JE, Brogden RN. Pentoxifylline (oxpentifylline). A review of its therapeutic efficacy in the management of perioheral vascular and cerebrovascular disorders. Drugs Aging. 1995;7:480–503. doi: 10.2165/00002512-199507060-00007. [DOI] [PubMed] [Google Scholar]
- Glauser MP, Heumann D, Baumgarther JD, Cohen J. Pathogenesis and potencial strategies for prevention and treatment of septic shock: an update. Clin. Infect. Dis. 1994;18(Suppl. 2):S205–S216. doi: 10.1093/clinids/18.supplement_2.s205. [DOI] [PubMed] [Google Scholar]
- Hardawat AM. Responses of the microcirculation during sepsis/septic shock. Crit. Care. Med. 1996;24:1072–1077. [Google Scholar]
- Hood SC, Moher D, Barber GG. Management of intermittent claudication with pentoxifylline: meta-analysis of randomized contrlled trials. Can. Med. Assoc. J. 1996;155(8):1053–1059. [PMC free article] [PubMed] [Google Scholar]
- Jewett A, Bonavida B. Pentoxifylline suppress interleukin 2 mediated activation of immature human natural killer ceels by inhibiting endogenous tumor necrosis factor alpha secretion. J. Clin. Immunol. 1994;14:31–38. doi: 10.1007/BF01541173. [DOI] [PubMed] [Google Scholar]
- Jilg M, Barsig J, Leist M, Kusters S, Volk H-D, Wendel A. Enhanced release of interleukin-10 and soluble tumor necrosis factor receptors as novel principles of methylxantine action in murine models of endotoxic shock. J. Pharmacol. Exp. Ther. 1996;278:421–431. [PubMed] [Google Scholar]
- Koch HP. Thalidomide and congeners as anti-inflammatory agents. In: Ellis GP, West GB, editors. Progress in Medicine Chemistry. New York: Elsevier Science Publishers; 1994. pp. 166–218. [Google Scholar]
- Krausz T, Cohen J. Shock. In: McGee J, Isaacson PG, Wright N A, editors. Oxford Textbook of Pathology, Principles of Pathology. 1. Vol. 1. New York: Oxford University Press; 1992. pp. 540–551. [Google Scholar]
- Lehmann V, Freudenberg MA, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J. Exp. Med. 1987;165:657. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesslauer W, Tabuchi H, Gentz R, et al. Recombinant soluble tumor necrosis factor receptor proteins protect mice from lipopolisaccaide-induced lethaly. Eur. J. Immunol. 1991;21:2883–2886. doi: 10.1002/eji.1830211134. [DOI] [PubMed] [Google Scholar]
- McBride WT, Armstrong MA, McBride SJ. Immunomodulation: an important concept in modern anaesthesia. Anaesthesia. 1996;51:465–473. doi: 10.1111/j.1365-2044.1996.tb07793.x. [DOI] [PubMed] [Google Scholar]
- Mohler KM, Sleath PR, Fitzner JN, et al. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- Neuner P, Klosner G, Schauer E, et al. Pentoxifylline in vivo down regulates of IL-1B, IL-6, IL-8 and tumour necrosis factor a by human peripheral blood mononuclear cells. Immunology. 1994;83:262. [PMC free article] [PubMed] [Google Scholar]
- Ostrosky L, Soto JL, Angeles V, et al. Effects of Pentoxifylline or Dexametasone in combination with Amphoterincin B in Experimental Murine Cerebral Cryptoccocosis. Evidence of Neuroexcitatory Pathogenic Mechanisms. Antimicrob. Agent Chemther. 1996;40:1194. doi: 10.1128/aac.40.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia G, Arrieta O, Ríos C, Altagracia M, Kravzov J, Sotelo J. Effect of thalidomide against different tumors in rodents. J. Exp. Therap. Oncol. 1998. in press. [DOI] [PubMed]
- Randall T. Thalidomide's back in the News, but in more favorable circumstances. J.A.M.A. 1990;263:1467–1468. [PubMed] [Google Scholar]
- Saez-Llorens X, Ramilo M, Mustafa J, et al. Pentoxifylline modulates meningeal inflammation in experimental bacterial meningitis. Antimicrob. Agents Chemother. 1994;34:837–843. doi: 10.1128/aac.34.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Rush B, Simonnian G, Murphy T, Hsieh J, Condon M. Thalidomide inhibits TNF response and increases survival following endotoxin inyection in rats. J. Surg. Res. 1996;63:143–146. doi: 10.1006/jsre.1996.0237. [DOI] [PubMed] [Google Scholar]
- Vadas P, Pruzanski W. Induction of group II phospholipasa A2 expression and pathogenesis of the sepsis syndrome. Circulatory Shock. 1993;39:160–167. [PubMed] [Google Scholar]


