Abstract
Background
Chronic kidney disease (CKD) is a growing problem among the elderly. Early detection is considered essential to ensure proper treatment and to avoid drug toxicity, but detection is challenging because elderly patients with CKD often have normal serum creatinine levels. We hypothesized that most cases of CKD in the elderly would go undetected, resulting in inappropriate prescribing.
Objective
To determine whether recognition of CKD is associated with more appropriate treatment
Design
Retrospective chart review
Participants
All patients aged ≥65 years with a measured serum creatinine in the past 3 years at 2 inner city academic health centers.
Measurements
Estimated glomerular filtration rate (eGFR) calculated using the Modified Diet in Renal Disease equation, and for patients with eGFR < 60, documentation of CKD by the provider, diagnostic testing, nephrology referral and prescription of appropriate or contraindicated medications.
Results
Of 814 patients with sufficient information to estimate eGFR, 192 (33%) had moderate (eGFR < 60 mL/min) and 5% had severe (eGFR < 30 mL/min) CKD. Providers identified 38% of moderate and 87% of severe CKD. Compared to patients without recognized CKD, recognized patients were more likely to receive an ACE/ARB (80% vs 61%, p = .001), a nephrology referral (58% vs 2%, p < .0001), or urine testing (75% vs 47%, p < .0001), and less likely to receive contraindicated medications (26% vs 40%, p = .013).
Conclusions
Physicians frequently fail to diagnose CKD in the elderly, leading to inappropriate treatment. Efforts should focus on helping physicians better identify patients with low GFR.
KEY WORDS: chronic kidney disease, diagnosis, creatinine, ace inhibitors, elderly
INTRODUCTION
Chronic kidney disease (CKD) is now recognized as having a major impact on public health, affecting 11% of the adult population, but it remains underdiagnosed, particularly in the elderly.1,2 The early recognition of kidney disease is important because medical interventions, including the use of angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), can slow the progression of disease.3–5 Detection is challenging because serum creatinine, although convenient to measure, performs poorly in diagnosing CKD in the elderly, for whom significant declines in glomerular filtration rate (GFR) can be obscured owing to the effects of age, weight, muscle mass, race, and sex.6 As a consequence, clinical guidelines by the National Kidney Foundation (NKF)7 and others8 call for screening for CKD using either the Cockcroft-Gault (CG)9 or Modified Diet in Renal Disease (MDRD)10 equations, which can be easily calculated, instead of serum creatinine. Of the two, the Modified Diet in Renal Disease (MDRD) equation appears to be more accurate for older patients,11–15 and is recommended by the NKF.
A few studies have demonstrated that primary care physicians relying on serum creatinine fail to diagnose CKD in elderly patients,16,17 and that diagnostic rates can be improved by providing estimated GFR (eGFR) based on either the CG or MDRD equations.17–19 Whether such recognition leads to changes in physician practice which may slow the progression of chronic kidney disease is unclear. Specifically, if CKD is recognized, are physicians more likely to prescribe ACE inhibitors or ARBs, and are they less likely to prescribe drugs that are nephrotoxic or have increased side effects in CKD? Older patients are particularly vulnerable to medication side effects, and a GFR < 50 is recognized as a risk factor for adverse drug events.20 Moreover, recent studies demonstrate that older patients are both overprescribed contraindicated drugs21 and underprescribed ACE inhibitors for hypertensive and diabetic renal disease.22
We hypothesized that 4 years after publication of the NKF guidelines and initiation of the National Kidney Disease Education Project (NKDEP) aimed at raising awareness among primary care providers,23 renal disease would still be underdiagnosed in the primary care setting, especially for older patients with near normal serum creatinine, and that failure to recognize kidney disease would lead to underprescribing of renal protective medicines and inappropriate prescribing of other, harmful medications.
METHODS
Setting
We reviewed the charts of all patients 65 years or older as of March 1, 2006, at 2 inner city academic health care clinics in Springfield, MA. The 2 clinics are the main teaching sites for the Internal Medicine residency program at Baystate Medical Center, and serve a population of mostly minority, low-income urban patients. Each center has approximately 30,000 adult visits per year and approximately 450 patients over age 65 years, and both use the same central laboratory. Subjects were identified by age through a computer search of the 2 outpatient databases. Subjects were excluded if they had no serum creatinine or weight recorded in the 3 years before enrollment or were currently on hemodialysis. The chart review was completed on June 1, 2007.
Data Collection
Each patient record was reviewed by 1 of 4 physicians or a medical student. We reviewed paper charts and the electronic medical record and recorded the following information for each patient: age, gender, race, ethnicity, language preference, clinic, insurance status, height, weight, most recent blood pressure, and highest serum creatinine level recorded in past 12 months (or the most recent value if >12 months). Since 2004, serum creatinine at Baystate Medical Center is calibrated against the MDRD standard to enhance accuracy.24 For patients with an eGFR less than 60 mL/min by the MDRD equation, we recorded the following additional information: co-morbid illnesses associated with chronic renal disease (congestive heart failure, diabetes, hypertension, connective tissue diseases, inflammatory diseases, autoimmune diseases, and acute renal failure), medications that are contraindicated or require dosage adjustment for reduced GFR (NSAIDS, meperedine, propoxyphene, metformin, glyburide, digoxin >0.125 mg, atenolol >50 mg, lithium, ranitidine >150 mg, gabapentin >1,400 mg, and allopurinol >200 mg), ACE inhibitors and ARBs, laboratory data (hemoglobin, calcium, phosphate and albumin, urinalysis, measured creatinine clearance, microalbumin, 24-hour protein excretion), and radiological studies including renal ultrasound, renal computed tomography (CT) scan, and renal MRI/MRA. Finally, for each patient we reviewed the problem list and office visit notes for any indication by the provider that the patient had CKD (e.g., renal insufficiency, renal failure, nephropathy, kidney disease, kidney failure, increased creatinine, or decreased GFR).
Outcomes
Chronic kidney disease was defined by an eGFR of <60 mL/min by the 4-variable MDRD equation (=175* serum creatinine−1.154*Age−0.203*0.742 [for females]*1.21[for African Americans]).24 Outcomes for patients with CKD included identification of CKD by the provider, ACE/ARB use, and the use of medications or dosages, which are contraindicated in CKD (“inappropriate drugs”). Other outcomes included nephrology referral, diagnostic urine testing, serum calcium, hemoglobin level, diagnostic imaging, and systolic blood pressure <130.
Data Analysis
Glomerular filtration rate was estimated for all patients with available data. Moderate CKD was defined as eGFR between 30 and 60 mL/min and severe CKD as eGFR <30 mL/min. Descriptive statistics were computed for all patients with complete data, as well as by level of CKD. Chi-square tests were used to evaluate associations of patient characteristics with level of CKD. Among patients with moderate or severe CKD, associations of patient characteristics with physician recognition of renal insufficiency were evaluated by chi-square tests or 2-sample t tests. In addition, we evaluated association of selected patient characteristics and physician prescribing practice with severe versus less severe CKD by chi-square. Logistic regression models were developed for the outcomes ACE/ARB prescribed and any inappropriate drug prescribed among patients with CKD. All available patient characteristics were included in the models. Pairwise interactions were evaluated and retained with p < .10. Odds ratios with 95% confidence intervals are reported.
RESULTS
We reviewed records for 866 patients aged 65 years and older at the 2 clinics. Fifty-two patients were excluded owing to insufficient information to estimate GFR, or because they were on dialysis. Excluded patients did not differ by age, sex, or race from included patients, but were more likely to attend 1 clinic than the other. Patient characteristics appear in Table 1. Overall, 33% of patients had CKD and 6% had severe CKD. CKD was most common in patients aged 75–84 years, with 41% having an eGFR <60 mL/min. Older patients were more likely to have moderate, but not severe, CKD. Gender, race, and language preference were not significantly associated with CKD. Patients with CKD had a higher mean serum creatinine than those without, but 23% of patients with CKD had a serum creatinine within the laboratory limit of normal (≤1.2 mg/dL for males and ≤1.1 mg/dL for females). All patients with severe CKD (eGFR <30 mL/min) had abnormal serum creatinine.
Table 1.
Association of Patient Characteristics with Creatinine Clearance (eGFR) as Estimated by MDRD Equation
| Patient Characteristics | Overall n (%) | eGFR ≥ 60 n (%) | 30≤eGFR < 60 n (%) | eGFR < 30 n (%) | p value |
|---|---|---|---|---|---|
| Overall | 814 (100) | 549 (67) | 220 (27) | 45 (6) | |
| Age Group | .033 | ||||
| 65–74 years | 539 (66) | 383 (70) | 131 (60) | 25 (56) | |
| 75–84 years | 219 (27) | 130 (24) | 72 (33) | 17 (38) | |
| 85+ years | 56 (7) | 36 (7) | 17 (8) | 3 (6) | |
| Gender | .75 | ||||
| Male | 307 (38) | 211 (38) | 81 (37) | 15 (33) | |
| Female | 507 (62) | 338 (62) | 139 (63) | 30 (67) | |
| Race/Ethnicity* | .37 | ||||
| White | 213 (26) | 138 (25) | 63 (29) | 12 (27) | |
| Black | 329 (41) | 235 (43) | 75 (34) | 19 (43) | |
| Hispanic | 250 (31) | 164 (30) | 75 (34) | 11 (25) | |
| Other | 19 (2) | 11 (2) | 6 (3) | 2 (5) | |
| Language | .19 | ||||
| Non-Spanish speaking | 587 (72) | 403 (73) | 149 (68) | 35 (78) | |
| Spanish speaking | 227 (28) | 146 (27) | 71 (32) | 10 (22) | |
| Location | .022 | ||||
| Clinic A | 377 (46) | 236 (43) | 118 (54) | 23 (51) | |
| Clinic B | 437 (54) | 146 (57) | 102 (46) | 22 (49) | |
| Serum Creatinine | |||||
| Mean (SD) | 1.14 (0.78) | 0.85 (0.19) | 1.40 (0.33) | 3.43 (1.92) | |
| Median (Q1, Q3) | 1.0 (0.8, 1.2) | 0.8 (0.7, 1.0) | 1.3 (1.2, 1.6) | 2.5 (2.1, 4.3) | |
| High serum creatinine† | 214 (26) | 11 (2) | 158 (72) | 45 (100) | <.0001 |
*Missing Race/Ethnicity on 3 patients
†High serum creatinine is defined as >1.2 mg/dL for males and >1.1 mg/dL for females
Identifying Renal Insufficiency
Providers were more likely to identify CKD among men, African Americans, English-speaking patients, and those with diabetes or hypertension or both (Table 2). Only 14% of patients with CKD were identified if they had neither diabetes nor hypertension. Patients identified by providers as having CKD had significantly higher serum creatinine (2.2 mg/dL vs 1.4 mg/dL). Providers successfully identified renal insufficiency in 57% of cases with elevated serum creatinine, but only 9% of cases with normal serum creatinine (p < .0001). In multivariable analysis, only elevated serum creatinine was associated with provider recognition of CKD (OR 11.2, 95% CI 4.1 to 30.7). Overall, providers identified CKD in 38% of patients with eGFR <60 mL/min and 87% of patients with eGFR <30 mL/min (p < .0001).
Table 2.
Association of Patient Characteristics with Recognition of CKD among Patients with CKD
| Patient Characteristics | CKD recognized by provider n (row %) | CKD not recognized by provider n (row %) | p value |
|---|---|---|---|
| Overall | 121 (46) | 142 (54) | |
| Age Group | .30 | ||
| 65–74 years of age | 77 (50) | 77 (50) | |
| 75–84 years of age | 36 (40) | 53 (60) | |
| 85+ years of age | 8 (40) | 12 (60) | |
| Gender | 0.017 | ||
| Male | 53 (56) | 42 (44) | |
| Female | 68 (40) | 100 (60) | |
| Race/Ethnicity | 0.042 | ||
| White | 32 (43) | 42 (57) | |
| Black | 52 (56) | 41 (44) | |
| Hispanic | 31 (36) | 55 (64) | |
| Other | 5 (63) | 3 (37) | |
| Language | 0.052 | ||
| Non-Spanish speaking | 91 (50) | 91 (50) | |
| Spanish speaking | 30 (37) | 51 (63) | |
| Diagnoses | 0.0031 | ||
| Diabetes only | 5 (71) | 2 (29) | |
| Hypertension only | 41 (43) | 55 (57) | |
| Diabetes and Hypertension | 70 (53) | 63 (47) | |
| Neither | 3 (14) | 19 (86) | |
| Location | 0.037 | ||
| Clinic A | 56 (40) | 84 (60) | |
| Clinic B | 65 (53) | 58 (47) | |
| Serum Creatinine (units) | |||
| Mean (SD) | 2.22 (1.49) | 1.36 (0.39) | |
| Median (q1, q3) | 1.70 (1.40, 2.20) | 1.30 (1.10, 1.50) | <.0001 |
| Creatinine Clearance by MDRD | |||
| Mean (SD) | 34.7 (13.0) | 46.9 (9.7) | |
| Median (q1, q3) | 37.1 (26.9,43.6) | 49.0 (39.3,54.3) | <.0001 |
| High Serum creatinine | <.0001 | ||
| Yes | 114 (56) | 88 (44) | |
| No | 7 (11) | 54 (89) |
Medication Prescribing
Providers prescribed inappropriate medications to 33% of patients with renal insufficiency. The most common inappropriate medications prescribed were NSAIDs, metformin, and atenolol at a dose >50 mg. Patients with identified CKD were both less likely to receive an inappropriate medication and more likely to receive an ACE/ARB than patients whose CKD was not identified (Fig. 1). Compared to patients with moderate CKD, those with severe CKD were less likely to receive an inappropriate medication (11% vs 38%, p < .001), but equally likely to receive an ACE or ARB (73% vs 69%, p = .57).
Figure 1.
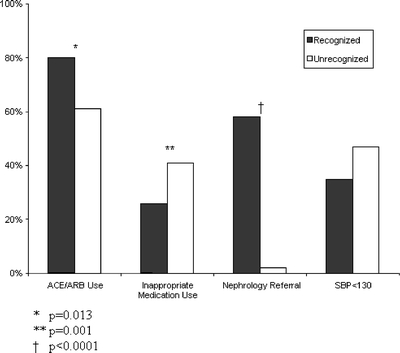
Key care measures in recognized and unrecognized renal insufficiency among patients with eGFR < 60 mL/min.
In multivariable analyses, a diagnosis of diabetes or hypertension was significantly associated with ACE/ARB prescribing, whereas advanced age was associated with lower inappropriate drug prescription (Table 3). Only physician identification of CKD, however, was significantly associated with both. Serum creatinine was not associated with either ACE/ARB prescribing or inappropriate medication use.
Table 3.
Multivariable Prediction Prescribing for Patients with eGFR < 60 mL/min
| Groups | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Appropriate prescribing (ACE/ARB) | ||
| Age Group | ||
| 65–74 years | 1 | |
| 75–84 years | 0.85 | (0.54, 1.35) |
| 85+ years | 0.72 | (0.45, 1.15) |
| Gender | ||
| Female | 0.63 | (0.32, 1.24) |
| Male | 1 | |
| Race/Ethnicity | ||
| White | 1 | |
| Black | 1.38 | (0.65, 2.91) |
| Hispanic | 1.61 | (0.75, 3.47) |
| Other | 2.78 | (0.31, 25.25) |
| Diagnoses | ||
| Diabetes | 2.89 | (1.52, 5.33) |
| Hypertension | 2.91 | (1.18, 7.20) |
| CKD identified | 2.35 | (1.20, 4.57) |
| Clinic | ||
| Clinic A | 1 | |
| Clinic B | 0.77 | (0.42,1.42) |
| High serum creatinine | 0.66 | (0.30,1.46) |
| Inappropriate Medications | ||
| Age Group | ||
| 65–74 years | 1 | |
| 75–84 years | 0.54 | (0.34, 0.88) |
| 85+ years | 0.29 | (0.18, 0.49) |
| Gender | ||
| Female | 1.30 | (0.70, 2.41) |
| Male | 1 | |
| Race/Ethnicity | ||
| White | 1 | |
| Black | 0.91 | (0.43, 1.93) |
| Hispanic | 1.38 | (0.68, 2.80) |
| Other | 3.95 | (0.76, 20.53) |
| Diagnoses | ||
| Diabetes | 1.65 | (0.91, 2.98) |
| Hypertension | 0.87 | (0.36, 2.14) |
| CKD Identified | 0.47 | (0.25, 0.88) |
| Clinic | ||
| Clinic A | 1 | |
| Clinic B | 0.74 | (0.42,1.31) |
| High serum creatinine | 0.79 | (0.38,1.63) |
Laboratory Testing and Referral
Of patients with eGFR < 60, 60% had diagnostic urine testing (14% had 24-hour urine, 37% had spot testing for microalbumin, and the remainder had urinalysis only), 82% had a hemoglobin level, and 60% had a serum calcium level measured. A minority had imaging studies, including renal ultrasound (22%) or CT/MRI (16%). Approximately one fourth was referred to a kidney specialist—20% of those with moderate and 64% of those with severe CKD (p < .0001). Patients with CKD identified were both more likely to be referred to a specialist, and to have any of the diagnostic tests, except hemoglobin and calcium levels (Table 4). There were no significant differences in hemoglobin, calcium, or blood pressure between patients with or without CKD identified.
Table 4.
Association of Referral and Testing with Recognition of CKD among Patients with CKD
| Intervention | CKD Recognized By Provider n (col %) | CKD Not Recognized By Provider n (col %) | p value |
|---|---|---|---|
| Nephrology referral | 69 (58) | 3 (2) | <.0001 |
| Serum Hemoglobin | |||
| Mean (SD) | 12.32 (1.78) | 12.37 (1.69) | |
| Median (q1,q3) | 12.10 (11.30,13.50) | 12.50 (11.60, 13.3) | .85 |
| Serum Hemoglobin measured | 93 (77) | 117 (82) | .26 |
| Serum Calcium Mean (SD) | 9.20 (1.50) | 9.26 (1.02) | .77 |
| Serum Calcium measured | 77 (64) | 80(56) | .23 |
| Systolic BP | |||
| Mean (std) | 141 (26.8) | 153 (144.1) | .50 |
| Systolic BP <130 | 26 (36) | 27 (47) | .23 |
| 24-hour Urine for GFR measured | 34 (51) | 2 (2) | <.0001 |
| Diagnostic urine test | 89 (75) | 66 (47) | <.0001 |
| Renal US | 46 (39) | 11 (8) | <.0001 |
| Renal MRI/A or CT | 32 (27) | 8 (6) | <.0001 |
Note: 2 patients missing data on recognition of CKD are excluded from analyses.
Discussion
Chronic kidney disease remains a common and underrecognized source of morbidity and mortality in the elderly. Identifying CKD is important because the use of ACE/ARB has been shown to slow the progression of renal disease,3–5 and a number of common medications are either contraindicated or require dosage adjustment in the setting of renal impairment. In addition, early referral to a kidney specialist has been linked to decreased mortality.25,26 In 2 inner city ambulatory general medicine clinics, we found that renal insufficiency among the elderly affected almost one third of patients, with 5% having severe renal insufficiency. Despite this, providers identified less than half of all cases. Patients who had normal serum creatinine or did not suffer from diabetes or hypertension were most likely to be missed. We found that when providers failed to identify CKD, they were more likely to prescribe inappropriate drugs and less likely to prescribe either ACE inhibitors or ARBs.
In an effort to improve early recognition of CKD and the implementation of secondary prevention strategies, in 2002 the National Kidney Disease Outcomes Quality Initiative (N/KDOQI) published evidence-based guidelines on recognizing and treating CKD.7 A year later, the NKDEP was launched to raise awareness of CKD among primary care providers and high-risk patients.23 Both efforts recommend the reporting of eGFR along with serum creatinine, and the NKDEP website provides an online GFR calculator and a downloadable PDA tool. Several years later, awareness of CKD diagnosis and treatment remains low. Few primary care clinicians have heard of the NKF guidelines or use eGFR for diagnosis.27–29
In addition, the premise that laboratory reporting of eGFR will improve recognition and treatment remains unproven. At least 3 studies have assessed the effects of eGFR reporting on clinician recognition and behavior.17–19 Two of these, which included only reporting, found only modest increases in physician recognition. A third study, which included an educational intervention as well, noted a 4-fold increase in detection rates.18 Given the small improvements in physician recognition seen with reporting alone, it may have been the educational intervention that improved recognition in this last study. If that is the case, emphasizing estimated GFR may not be enough. Indeed, the physicians in our study did not have automated reporting of eGFR, but still identified a high proportion of patients with CKD, perhaps because of the physicians’ participation in a training program.
The association between the recognition of CKD and improved management has been less studied. One large VA study found that providers identified only 10% of moderate and 45% of severe CKD, and that documentation of CKD was associated with ordering urinalysis, but not with ACE/ARB use or blood pressure control.17 In contrast, we found that our providers identified 46% of CKD and 87% of severe CKD, and that identification of CKD was associated with increased prescribing of ACE/ARB, decreased use of inappropriate medications, and increased referrals to a kidney specialist. One possible explanation for this difference is the method of determining whether physicians identified CKD. The VA study relied on ICD-9 codes, which may have underestimated physician recognition, whereas we reviewed physician notes.
Finally, we noted that ACE/ARB prescribing was associated with diabetes and hypertension, independent of recognized renal insufficiency. This may be the result of prescribing guidelines for diabetes and the reality that elderly patients with hypertension often require multiple agents to achieve acceptable blood pressure control. Because both diseases are known risk factors for renal disease, providers did a much better job identifying CKD in these populations. Conversely, they identified only 14% of CKD in patients with neither of these diagnoses. Educational interventions should therefore draw attention to identifying renal disease in patients without diabetes.
Our study has several limitations. First, it was a retrospective study and may contain unmeasured biases. Although we carefully reviewed the charts, we cannot know for sure that physicians were unaware of some patients’ renal failure; their practice patterns suggest that they were. Second, the population was limited to 2 sites within the same health care system and therefore may not be generalizable. Our patients did, however, represent a diverse population of age, ethnicity, and comorbid disease. Third, our definition of CKD was based on GFR estimated by the MDRD equation. Although this estimation is convenient and recommended by the NKDEP, classifying patients based on other measurements of GFR might have yielded different results. Changing the definitions, however, would not affect the relationship between physician recognition and behavior, which was independent of eGFR. Finally, we studied medication use, but not direct patient outcomes, such as health care utilization or mortality, and the benefits of ACE/ARB in the elderly, and especially the very old, have not been well documented. Nevertheless, the medications studied have already been shown to be beneficial in slowing the progression of renal disease in general and there is no reason to believe they would not be effective in our population. Consequently, the K/DOQI guidelines do not discriminate by age.7
In addition to the 239,836 Americans undergoing dialysis in 1999—a number that is expected to exceed 520,000 in 2010—renal dysfunction is emerging as an important risk factor for cardiovascular morbidity and mortality.30 The prevalence of endstage renal disease (ESRD) is rising fastest in the elderly, with 58% of newly diagnosed patients over age 65 years.31 The ability to identify renal insufficiency in the primary care setting is a vital prerequisite to implementing appropriate secondary prevention strategies. Nevertheless, the gap between evidence-based guidelines and clinical practice remains wide. It is not clear why, despite the NKDEP, K/DOQI guidelines have not penetrated primary care to the same extent as guidelines for hypertension, diabetes, or lipids, but educational efforts alone will not be enough. System-wide quality improvement interventions, working on multiple levels, and tailored to local conditions, will be necessary. In addition, more collaboration between the nephrology and primary care communities would be helpful. Despite the emphasis on primary care, most articles about the NKDEP, including the K/DOQI guidelines, appear in nephrology journals. This may explain why Nephrologists were 8 times more likely than Internists to be aware of the national guidelines.29
Our study demonstrates that primary care doctors do a relatively good job of managing CKD once they recognize it, but lack the tools to identify CKD when serum creatinine levels are “normal” or when patients do not have diabetes or hypertension. Whereas laboratory reporting of estimated GFR is an important first step to improving care, studies show that in most settings, such reporting is not enough to change physician practice in a significant way. Future studies should focus on educational and system interventions with greater potential to change the way we practice.
Acknowledgment
This study was supported by the Charlton Fund of Tufts University School of Medicine. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. Dr. Rothberg is the recipient of a Doris Duke Clinical Scientist Development Award.
In addition, we would like to acknowledge Ricky Wong for his help in collecting the data and Chris Kenwood and Yan Liu for their help with the statistical analysis.
Conflict of Interest None disclosed.
Footnotes
This paper was presented at the 2007 Annual Meeting of the American Geriatrics Society in Seattle, WA.
References
- 1.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–47. [DOI] [PubMed]
- 2.Clase CM, Garg AX, Kiberd BA. Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III). J Am Soc Nephrol. 2002;13:1338–49. [DOI] [PubMed]
- 3.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334:939–45. [DOI] [PubMed]
- 4.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–63. [DOI] [PubMed]
- 5.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–52. [DOI] [PubMed]
- 6.Papaioannou A, Ray JG, Ferko NC, Clarke JA, Campbell G, Adachi JD. Estimation of creatinine clearance in elderly persons in long-term care facilities. Am J Med. 2001;111:569–73. [DOI] [PubMed]
- 7.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–266. [PubMed]
- 8.Mathew TH. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust. 2005;183:138–41. [DOI] [PubMed]
- 9.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. [DOI] [PubMed]
- 11.Garg AX, Papaioannou A, Ferko N, Campbell G, Clarke JA, Ray JG. Estimating the prevalence of renal insufficiency in seniors requiring long-term care. Kidney Int. 2004;65:649–53. [DOI] [PubMed]
- 12.Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis. 2005;46:233–41. [DOI] [PubMed]
- 13.Van Den Noortgate NJ, Janssens WH, Delanghe JR, Afschrift MB, Lameire NH. Serum cystatin C concentration compared with other markers of glomerular filtration rate in the old old. J Am Geriatr Soc. 2002;50:1278–82. [DOI] [PubMed]
- 14.Cirillo M, Anastasio P, De Santo NG. Relationship of gender, age, and body mass index to errors in predicted kidney function. Nephrol Dial Transplant. 2005;20:1791–8. [DOI] [PubMed]
- 15.Fehrman-Ekholm I, Skeppholm L. Renal function in the elderly (>70 years old) measured by means of iohexol clearance, serum creatinine, serum urea and estimated clearance. Scand J Urol Nephrol. 2004;38:73–7. [DOI] [PubMed]
- 16.Swedko PJ, Clark HD, Paramsothy K, Akbari A. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med. 2003;163:356–60. [DOI] [PubMed]
- 17.Wyatt C, Konduri V, Eng J, Rohatgi R. Reporting of estimated GFR in the primary care clinic. Am J Kidney Dis. 2007;49:634–41. [DOI] [PubMed]
- 18.Akbari A, Swedko PJ, Clark HD, et al. Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch Intern Med. 2004;164:1788–92. [DOI] [PubMed]
- 19.Quartarolo JM, Thoelke M, Schafers SJ. Reporting of estimated glomerular filtration rate: effect on physician recognition of chronic kidney disease and prescribing practices for elderly hospitalized patients. J Hosp Med. 2007;2:74–8. [DOI] [PubMed]
- 20.Mackinnon NJ, Helper CD. Indicators of preventable drug-related morbidity in older adults 2. Use within a managed care organization. J Manag Care Pharm. 2003;9:134–41. [DOI] [PMC free article] [PubMed]
- 21.Curtis LH, Ostbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med. 2004;164:1621–5. [DOI] [PubMed]
- 22.Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients. Ann Intern Med. 2004;140:714–20. [DOI] [PubMed]
- 23.Hostetter TH, Lising M. National kidney disease education program. J Am Soc Nephrol. 2003;14:S114–6. [DOI] [PubMed]
- 24.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. [DOI] [PubMed]
- 25.Winkelmayer WC, Owen WF Jr., Levin R, Avorn J. A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol. 2003;14:486–92. [DOI] [PubMed]
- 26.Jones C, Roderick P, Harris S, Rogerson M. Decline in kidney function before and after nephrology referral and the effect on survival in moderate to advanced chronic kidney disease. Nephrol Dial Transplant. 2006;21:2133–43. [DOI] [PubMed]
- 27.Fox CH, Brooks A, Zayas LE, McClellan W, Murray B. Primary care physicians’ knowledge and practice patterns in the treatment of chronic kidney disease: an Upstate New York Practice-based Research Network (UNYNET) Study. J Am Board Fam Med. 2006;19:54–61. [DOI] [PubMed]
- 28.Lea JP, McClellan WM, Melcher C, Gladstone E, Hostetter T. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis. 2006;47:72–7. [DOI] [PubMed]
- 29.Boulware LE, Troll MU, Jaar BG, Myers DI, Powe NR. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis. 2006;48:192–204. [DOI] [PubMed]
- 30.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. [DOI] [PubMed]
- 31.Health Care Financing Administration. Network of New England I. End Stage Renal Disease 1999 Annual Report. New Haven, CT; 1999:84.


