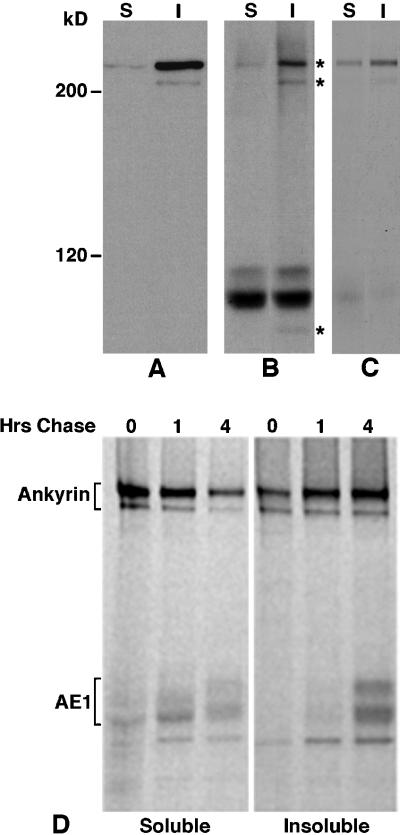
Figure 2.
Association of erythroid AE1 anion exchangers with ankyrin. Detergent soluble (S) and insoluble (I) fractions from 105 erythroid cells from a 10-d chicken embryo were analyzed on a 6% SDS polyacrylamide gel, transferred to nitrocellulose, and probed with an ankyrin-specific antibody. After washing, the blot was incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase, and immunoreactive species were detected by enhanced chemiluminescence (A). Erythroid cells from a 10-d-old chicken embryo were also pulsed for 15 min with 35S-Translabel and chased for 1 or 4 h in complete media. Aliquots of cells from the pulse and chase points were detergent lysed, and immunoprecipitates were prepared with the ankyrin-specific antibody from the detergent-soluble and insoluble fractions (D). Alternatively, AE1 anion exchanger immunoprecipitates were prepared from the detergent-soluble and -insoluble fractions of cells that had been pulsed for 15 min and chased for 4 h. The AE1 immunoprecipitates were either directly analyzed (B) or dissociated in SDS sample buffer and reprecipitated with the ankyrin-specific antibody (C). The immunoprecipitates were analyzed on a 6% SDS polyacrylamide gel, and labeled species were detected by fluorography. Asterisks to the right of panel B mark the 225-, 205-, and 85-kDa polypeptides that coprecipitate with AE1. Molecular weight markers to the left of panel A correspond to myosin (200 kDa), and β-galactosidase (120 kDa).