Figure 2.
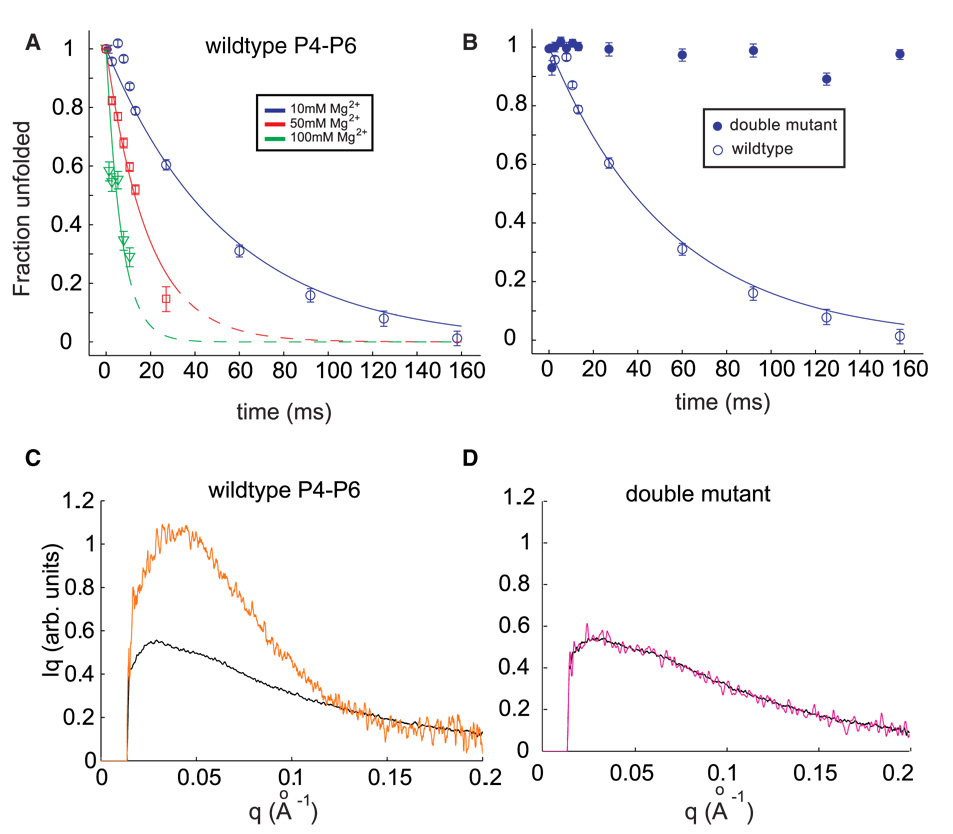
(A) Folding time courses for wild type P4–P6 at different magnesium concentrations. The coefficient Pu, representing the percentage of the unfolded scattering profile required to fit each time-resolved profile (see text) is shown following the addition of 10 mM Mg2+ (circles), 50 mM Mg2+ (squares) and 100 mM Mg2+ (triangles). Changes in the time courses can be explained by a model in which the flexibility of the J5-5a hinge varies with ionic strength. (B) The folding time course following addition of 10 mM Mg2+ for the wild type P4–P6 (open circles) is contrasted with the corresponding time course from the mutant that lacks the ability to form tertiary contacts. (C & D) Comparison of SAXS profiles, displayed as Iq vs. q, for the earliest (unfolded, black) and longest time point (t=160 ms, orange) following the addition of 10 mM Mg2+ to wild type (C) and mutant (D) P4–P6. While wild type P4–P6 folds to a structure compatible with the crystal structure (Figure 1B), no change in the conformation of the mutant is detected.
